Reports: DNI755562-DNI7: Synthesis and Design of Novel Metal-Doped Porous Organic Polymers: The Enhancement of pi-Complexation with Small Unsaturated Hydrocarbons
Psaras McGrier, PhD, The Ohio State University
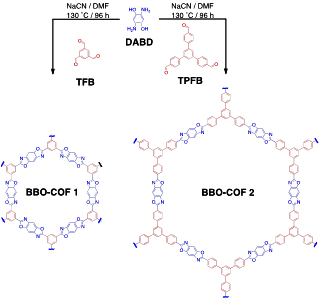
Developing novel synthetic strategies to construct crystalline polymeric materials with excellent chemical stability has become a challenging process. Thus far, we have reported the synthesis of two novel two-dimensional (2D) benzobisoxazole-linked covalent organic frameworks (BBO-COFs) utilizing C3-symmetric formyl- and C2-symmetric o-aminophenol-substituted molecular building blocks (Scheme 1). Polybenzoxazoles are a class of heterocyclic polymers that exhibit excellent thermal stability and superb chemical resistance. Recently, Yavuz and co-workers demonstrated that benzoxazole units can be incorporated into amorphous COPs and utilized for selective CO2 capture. However, the synthesis consists of a multi-step process, which involves annealing a silyl-protected prepolymer at 400 °C in its final step to form the polymer networks. In an effort to make this process more efficient, we have developed general synthetic route to form ordered 2D benzoxazole polymer networks using sodium cyanide as a catalyst.
Scheme 1. Synthesis of BBO-COF 1 and BBO-COF 2.
BBO-COF 1 and BBO-COF 2 were synthesized by first reacting 1,3,5-triformylbenzene (TFB) and 1,3,5-tris(4-formylphenyl)benzene (TFPB), respectively, with 2,5- diamino- 1,4-benzenediol dihydrochloride (DABD) in DMF 3 hours at -15 °C to ensure slow formation of the phenolic imine-linked intermediate and avoid rapid precipitation. Afterwards, the mixture was slowly brought to room temperature overnight before adding 1 equivalent of sodium cyanide dissolved in 0.2 mL of methanol to the mixture. The solution was then stirred under air at 130 °C for 4 days. BBO-COF 1 and 2 were obtained by filtration and washed with acetone to yield light brown crystalline solids that were insoluble in common organic solvents. The BBO-COFs were purified by soaking them in methanol and acetone over a 48 h period to remove unreacted monomers and then dried under vacuum. The formation of the BBO linkage is believed to proceed through the following three-step mechanism: 1) the formation of a phenolic imine-linked intermediate, 2) subsequent addition of cyanide to the imine to induce ring closure and formation of a benzoxazoline intermediate, and finally 3) aerobic oxidation of the benzoxazoline intermediate under air to promote the formation of the BBO linkage. The BBO-COFs were characterized by Fourier transform infrared (FT-IR) spectroscopy, 13C cross-polarization magic angle spinning (CP-MAS) NMR, and powder X-ray diffraction (PXRD).
Nitrogen gas isotherms revealed that BBO-COFs exhibit surface areas as high as 1106 m2 g-1. The BBO-COFs also exhibit CO2 uptake capacities as high as 150 mg g-1. This work was recently published in ACS Macro Letters. It should be noted that this research highlights the first example of utilizing cyanide as a catalyst to construct ordered 2D BBO-COFs, and establishes a new linkage for the COF community. The research also puts the PI and the graduate students involved with the project in a position to utilize this chemistry to develop BBO-COFs for applications related to sensory, organic electronic, and gas separations (e.g., small olefin/paraffin separations).
In addition, we have been able to utilize pi-conjugated dehydrobenzoannulene (DBA) units to construct luminescent 2D COFs. DBAs are triangular shaped macrocycles that have the ability to bind low oxidations state metals. We have been able to show that these monomers can be used to make multiple-component COFs (i.e., polymeric systems that contain more than two different monomers). Multiple component COFs are believed to be important for not only increasing the structural diversity of COFs, but also for providing access to ordered polymeric materials with unique separation, optoelectronic, and gas adsorption properties. This work provided the first example of using DBA monomers to create multiple component COFs and was recently published in the Journal of the American Chemical Society. We are now focusing our efforts on 1) constructing chemically stable BBO-linked DBA-COFs, and 2) utilizing the DBA units of these crystalline polymeric systems to bind metals, which could enhance their gas adsorption properties.