Reports: ND753960-ND7: Well-Defined Polyrings via Diyne-Functionalized Cyclic Monomers
Chong Cheng, PhD, State University of New York at Buffalo
The goal of this project is to establish a method for the synthesis of well-defined polyrings with a sizable ring per repeat unit, which are model polymers ideal for the study of the effect of side ring structures on polymer properties. The spacers between backbone and side rings of polymer rings should be either absent or very short, in order to eliminate or reduce the effect from linear portions of side groups. In the second grant year, substantial synthetic efforts were continuously made in the PI’s laboratory with two specific aims, including 1) to synthesize cyclic diyne monomers with oligo(ethylene glycol)-based rings and study metathesis polymerization of these monomers, and 2) to synthesize cyclic diyne monomers with crown ether rings and study metathesis polymerization of these monomers. The two specific aims correspond to two different structural designs of polyrings, respectively. In the first type of polyrings, the side rings have pseudo-crown ether structures and there are no spacers between polymer backbone and side rings. In the second type of polyrings, there are very short spacers between polymer backbone and the crown ether side rings.
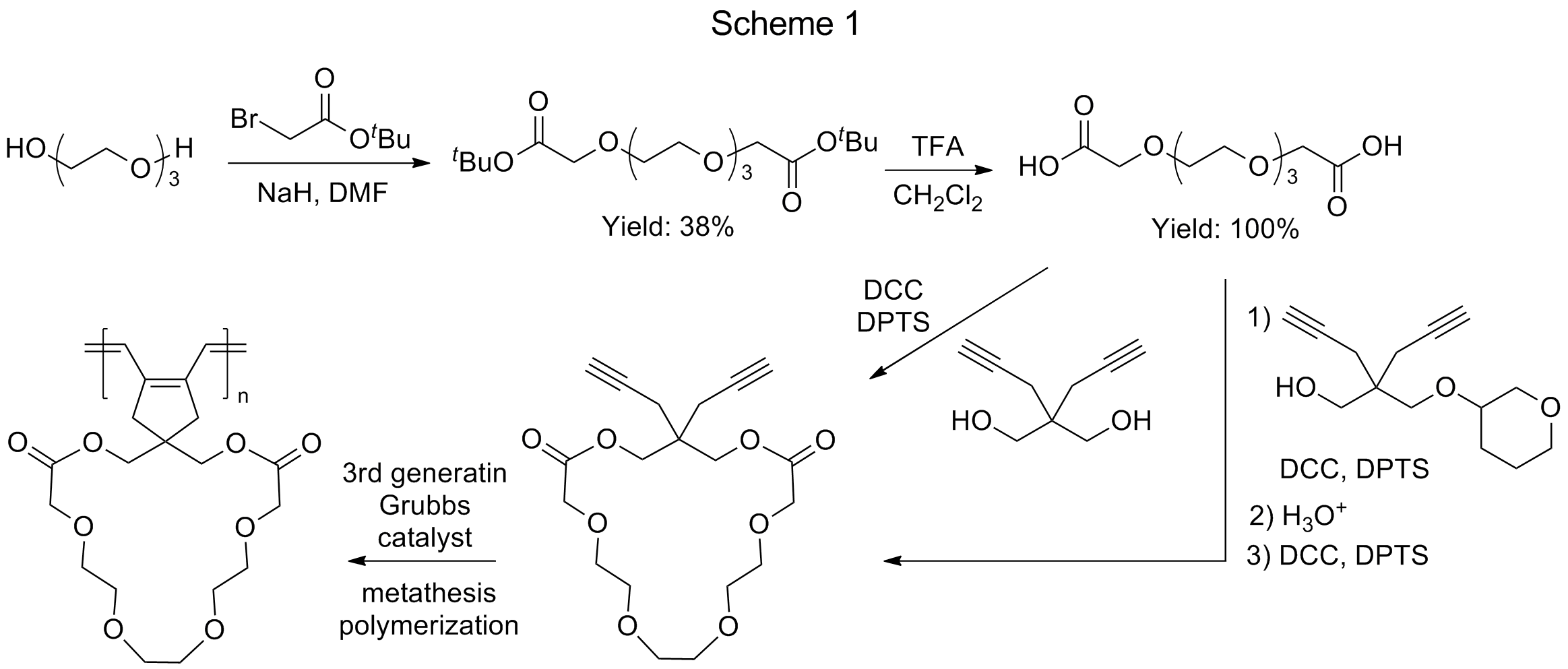
To synthesize the polyrings with pseudo-crown ether side rings, 3,6,9,12-tetraoxatetradecane-1,14-dioic acid, as a representative diacid-functionalized oligo(ethylene oxide) (OEG), was prepared at first (Scheme 1). Based on a literature approach,1 triethylene glycol was treated with excess amounts of t-butyl bromoacetate (2.5 eq) and sodium hydride (2.5 eq) in dry THF at 0°C and then warmed up to room temperature for overnight. The resulting OEG diester was obtained in 38% yield after separation using column chromatography. It was further treated with trifluoroacetic acid (TFA) in dichloromethane, to give OEG diacid in quantitative yield. Subsequent synthesis of OEG-based diyne monomer was investigated using two different routes. In the first route, one-step bimolecular ring-closure was conducted by the esterification reaction between the OEG diacid with a diyne-diol, 4,4’-bis(hydroxymethyl)-1,6-heptadiyne. In the second route, the OEG diacid reacted with the mono-protected diyne-diol, and the resulting monoesterified product was deprotected and used for unimolecular ring-closure reaction. However, no matter which route was used, separation of the final OEG-based diyne monomer from reaction mixtures was difficult. Once the separation issue is solved through steady efforts made by the PI’s lab, the resulting OEG-based diyne monomer will be obtained and its metathesis polymerization will be performed for the synthesis of polyrings.
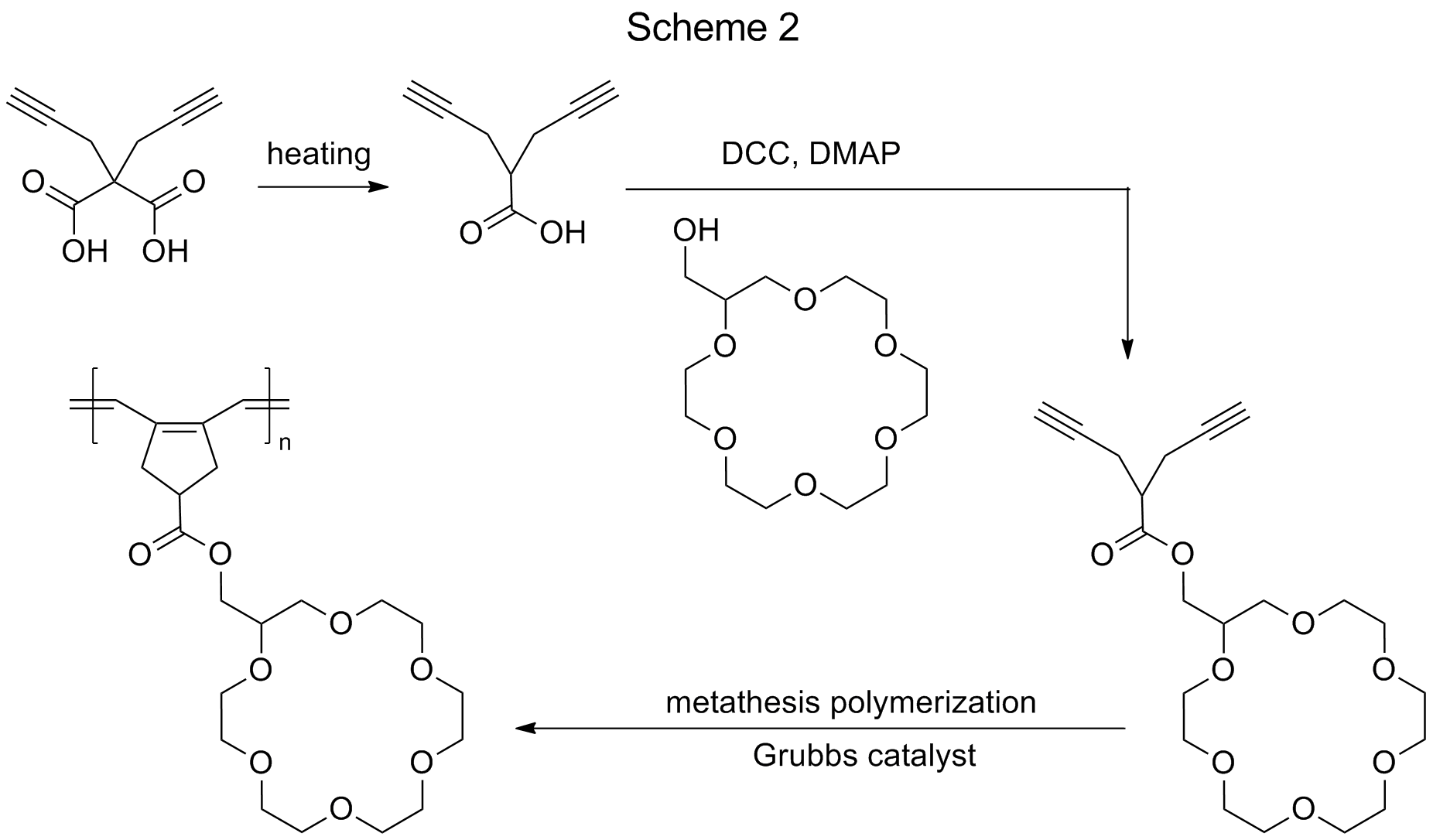
As an alternative option to access polyrings, crown ether-based diyne monomer were designed to afford polyrings via metathesis polymerization. An 18-crown-6-based diyne monomer was synthesized at first (Scheme 2). Following a reference approach,2 2,2-di(prop-2-ynyl)propan-1,3-dioic acid was heated at 135 °C to give 2-(2-propynyl)-4-pentynoic acid in 81% yield. The esterification reaction of 2-(2-propynyl)-4-pentynoic acid (1.5 eq) with 2-hydroxymethyl-18-crown-6 (from Aldrich) (1 eq) was conducted using dicyclohexylcarbodiimide (DCC) (1.5 eq) and 4-dimethylaminopyridine (DMAP) (0.1 eq) as catalysts in dry THF for a 3-day reaction time. The resulting diyne monomer was obtained in 50% yield. Metathesis polymerization of the monomer for the synthesis well-defined polyrings carrying 18-crown-6 side rings with short -COOCH2- spacers currently is ongoing, and the results will be reported in next annual report.
In summary, synthetic routes for the preparation of cyclic diyne monomers were continuously investigated in the second grant year. Although difficulties in the synthesis of a diyne monomer with a pseudo-crown ether ring were experienced, successful synthesis of a diyne monomer with 18-crown-6 ring was achieved. In the next grant year (in no-cost extension), we plan to 1) complete the synthesis of diyne monomer with a pseudo-crown ether ring, and 2) study all of the diyne monomers for the preparation of homopolymers and block copolymers.
References:
1) Wittmann, V.; Takayama, S.; Gong, K. W.; Weitz-Schmidt, H.; Wong, C.-H. J. Org. Chem. 1998, 63, 5137-5143.
2) Atkinson, R. S.; Grimshire, M. J., J. Chem. Soc. Perkin Trans. I 1986, 1215-1224.