Reports: UNI353848-UNI3: Development of Homogeneous Ru(II) Olefin Hydroarylation Catalysts Supported by Oxy-Anionic Functionalized Bis(pyrazolyl)alkane Ligands
Brandon Quillian, PhD, Armstrong State University
Project Objective: The petroleum industry has long been interested in catalytic carbon-carbon bond formation technologies for the conversion of raw materials into value-added materials. While the Friedel-Crafts reaction has been a preferred method for the production of alkylarenes, its multitude of drawbacks (waste, high energy consumption, non-selectivity) has caused scientist to search for better alternatives. Many of these exploits have been focused on transition metal-mediated C-H activation technologies that operate at reduced temperatures with greater selectivity.
We have joined this pursuit by developing homogeneous Ru(II) olefin hydroarylation (Addition of arene C-H bonds across carbon-carbon double bonds) catalysts supported by oxy-anionic functionalized bis(pyrazolyl)alkane ligands in an effort to assess the effects of oxygen-to-metal π-donation on the catalytic cycle. Because oxygen coordinating ligands are typically deemed inappropriate for use in catalytic processes involving saturated, d6-octahedral, late transition metal systems due to d-π orbital conflict, another aim of this project was to assess the stability of the Ru−O bond to provide evidence on these ligand variants’ feasibility in C−H activation reactions. Herein, we present our progress on the preparation of new ruthenium complexes and our future efforts.
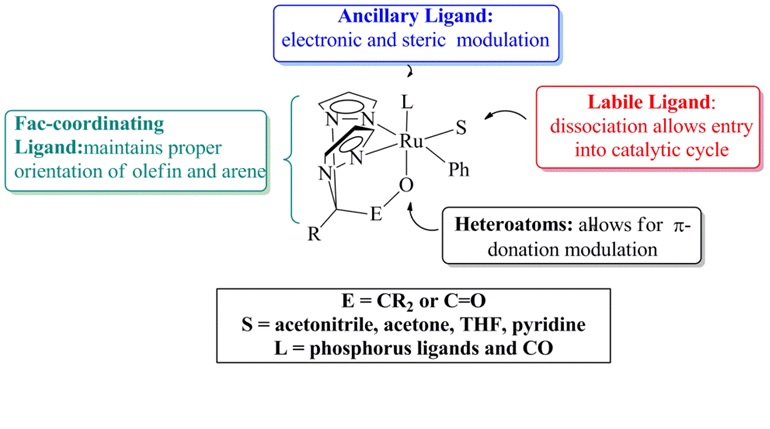
Research progress: We have been attempting to prepare Ru(II) complexes supported by bis(pyrazolyl)acetate ligands, phenyl group and ancillary ligands (Figure 1).
Figure 1. Prototypical features of a Ru(II) catalyst supported by κ3-N,N,O-oxy-anionic bis(pyrazolyl)methane ligands
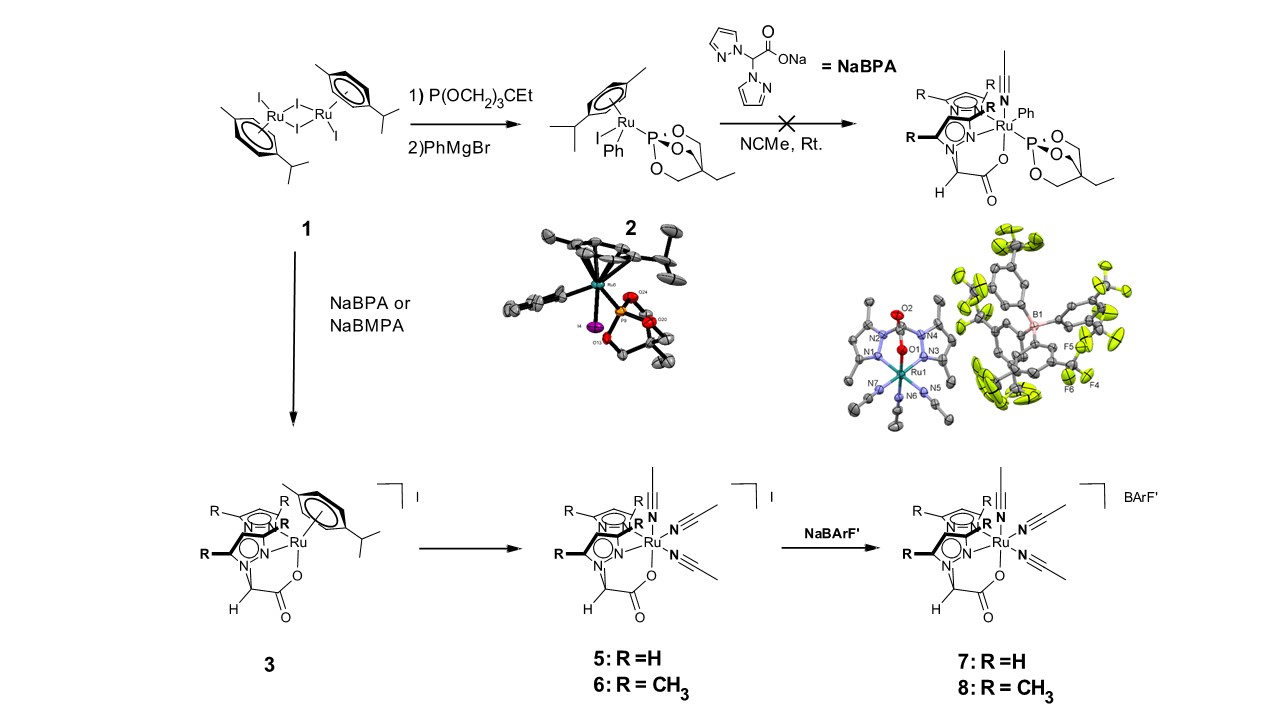
We chose [(η6-p-cyemene)RuI2]2 (1) as an entry point into these systems because of the facile reactivity of the Ru‑I bond with phenylating reagents. We have synthesized a viable intermediate, (η6-p-cyemene)RuI{P(OCH2)3CEt}(Ph) (2), that may serve as a synthon for our final pre-catalyst by reacting the staring material, 1, with a phosphorus donor-ligand followed by phenylation (Scheme 1). Unfortunately, our efforts to replace the η6-p-cyemene ligand of 2 with BPA to give our desire complex failed.
Scheme 1. Routes to prepare catalyst precursor; ultimate preparation of tris(acetonitrile)ruthenium complexes.
We then pursued an alternate approach by first attaching the BPA ligand to the ruthenium center of 1 to produce [(BPA)Ru(η6-p-cyemene)]I (3), followed by displacement of the cymene ligand with acetonitrile (NCMe) to produce (BPA)RuI(NCMe)2 (4; not shown). However, 4 is not formed, but instead the cationic, tris-acetonitrile Ru(II) complex, [(BPA)Ru(NCMe)3]I (5) (Scheme 1) is afforded. For the sake of structural-activity relationship studies, we also prepared the more sterically encumbered complex [(BMPA)Ru(NCMe)3]I (6) (BMPA = bis(3,5-dimethylpyrazoly)acetate). The solubility of 5 and 6 dramatically improved with swapping their iodide anion with BArFˊ (BArFˊ= tetrakis[3,5-bis(trifluoromethyl)phenylborate]), yielding [(BPA)Ru(NCMe)3][BArFˊ] (7) and [(BMPA)Ru(NCMe)3][BArFˊ] (8), respectively. While the reaction of 7 with phenylmagnesium bromide showed promising reactivity to suggest formation of Ru-Ph bonds, the reaction was not clean.
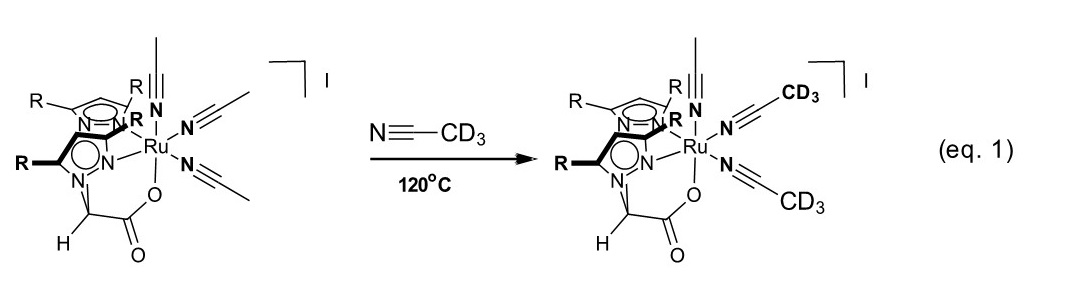
We examined acetonitrile dissociation rates of 7 and 8 to assess their lability and the stability of the Ru−O bond. These complexes are remarkably stable at temperatures over 120°C, suggesting the ligands are feasible for olefin hydroarylation studies. Moreover, these studies corroborated our hypothesis that the acetonitrile ligands would dissociate more readily due to the metal-oxygen d-π conflict. Indeed, we found the acetonitrile ligands cis to the Ru−O bond are significant more labile than that which is trans (eq. 1). Due to their structural similarity, complexes 7 and 8 may show similar reactivity to very versatile catalysts [Cp#RuNCMe][PF6] (Cp# = Cp; C5H5- or Cp*; C5(CH3)5-). We will submit an article highlighting the preparation of 7 and 8 and its reaction chemistry.
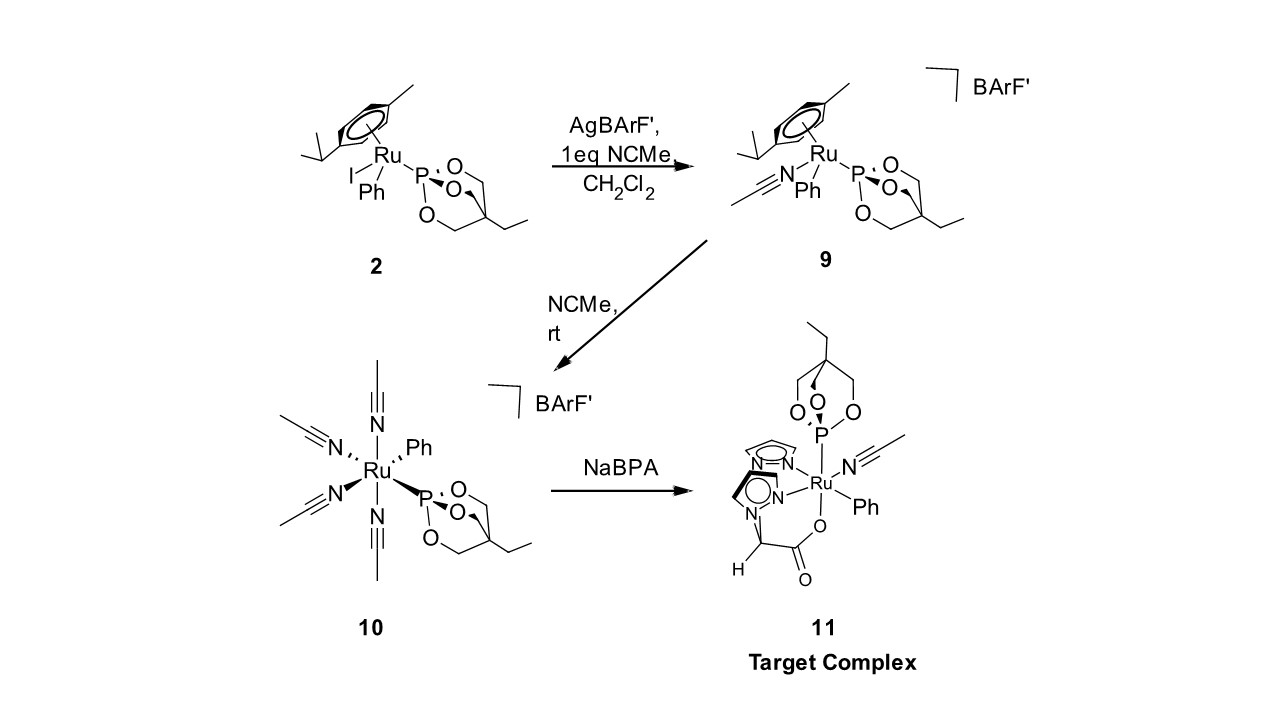
We have also prepared [(η6-p-cyemene)RuPh{P(OCH2)3CEt}(NCMe)][BArF’] (9); a synthon that has all of the hallmarks of a potential olefin hydroarylation catalyst (Scheme 2). However, we serendipitously discovered that allowing 9 to rest in an acetonitrile solution provided the tetra(acetonitrile)ruthenium(II) complex [(NCMe)4RuPh{P(OCH2)3CEt}][BArF’] (10). Reaction of 10 with NaBPA provides (BPA)Ru Ph{P(OCH2)3CEt}(NCME) (11) fairly cleanly. This compound represents our final catalyst precursor. We have only prepared 11 in NMR scale reactions and will begin scaled-up reactions to produce the complex in quantities necessary to begin our catalytic studies in the near future.
Scheme 2. Reaction sequence for preparation target complex (BPA)Ru Ph{P(OCH2)3CEt}(NCME) (11)
Faculty and Student Impact:
I have mentored 11 students in research projects funded or partially funded by the ACS-PRF grant. Three students were directly supported with ACS funds over two summer semesters (one student twice). Support for these research projects has resulted in the publication of three peer-reviewed articles and four additional articles in preparation. My students presented 10 posters at ACS National and Regional Meetings. One of the posters was invited to the SCIMIX Function at the 66th Southeast Regional Meeting of the American Chemical Society, Nashville, TN. These projects have afforded several opportunities for my students that have further aided them in their career pursuits. Every student who has entered my laboratory has either graduated or is on-track to graduate soon. This cohort of students has spanned broad demographics, including eight females and four minority students. John Haddock graduated in 2016 and is currently attending graduate school in chemistry at Florida State University. Macy McNair graduated in 2015 and just completed her first year at Morehouse Medical School. Mrinali Sharma is a Masters student in the Biomedical Science program at Barry University, Miami. Alexis Fields has several accolades relevant to her research pursuits. She recently was named an ACS-Scholar, a Georgia Power Scholar, and a Dean Research Scholar. She has plans to attend Graduate school in biochemistry in 2017. She will continue to push the project forward in 2016-2017 Academic year.
PI Impact: The ACS-PRF grant has had a tremendous impact on the PI’s professional and scholarly development. While this project is not nearly complete, it has provided preliminary results that will be included in future grant proposals to further support this work. Three research articles are currently being prepared to disseminate the results of this project. Moreover, there were a number of unexpected results that led to publications and will lead to an additional publication in the near future. Indeed, these tangential results have blossomed into an entirely new field for the PI to explore in the near future.