Reports: UNI153776-UNI1: Development of a Palladium-Catalyzed Oxidative Aryl Transfer Reaction
William Brenzovich, PhD, Roanoke College
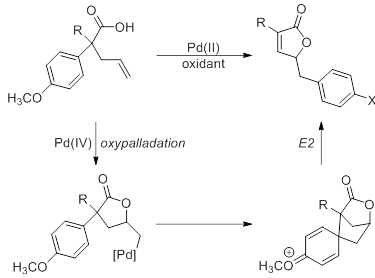
The major area of chemical research in our lab has been in the development and exploration of reactions catalyzed by metals in a high oxidation state. Palladium-catalyzed reactions have proven invaluable in the search for new and interesting reactions. Our lab’s specific aim is the study of a novel reaction catalyzed by palladium(IV) that involves the transfer of an aryl group. This reaction is unprecedented under palladium-catalysis and provides efficient access to an interesting structural motif, while offering insight into the reactivity of high-oxidation state palladium. Our lab is working to explore the scope and mechanism of this reaction through careful examination of the steric and electronic requirements of the system. The information garnered from this project will be used to inform the development of additional new and novel palladium-catalyzed transformations.
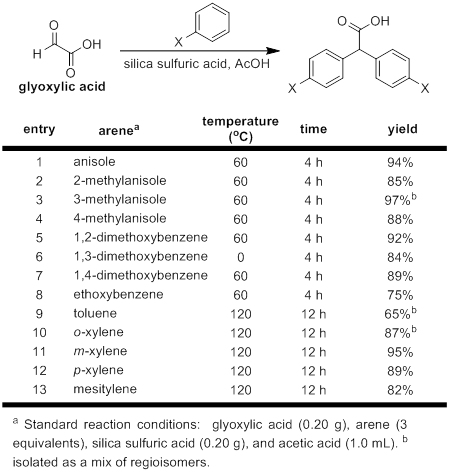
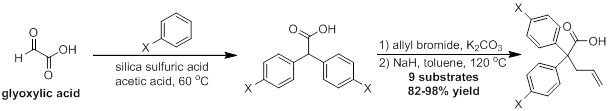
When investigating the synthesis of the diarylacetic acid starting materials, we developed several improvements for the previously available literature procedures. Using a more cost-effective and green heterogeneous catalyst, we have developed a more efficient synthesis for this interesting class of molecules. This new protocol proved to be both high yielding and ammenable to a variety of electron-rich arene substrates, including even sterically encumbered arenes such as mesitylene. In addition, we have been able to successfully apply this procedure for the synthesis of a variety of unsymmetric diarylacetic acids starting from mandelic acid derivatives. This reusable catalyst system has a variety of different potential applications that will be subject of future work within our laboratory. This work resulted in a publication in the spring of 2016.
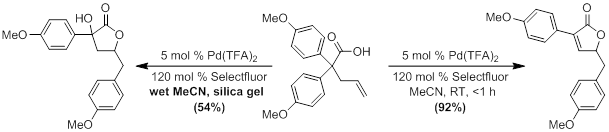
In previous years, we have optimized the synthesis of the substrate and the reaction conditions for the palladium-catalyzed reaction. Substrates are synthesized using the diarylacetic acids from the previously mentioned diarylacetic acids in a two-step protocol. Optimized conditions for both the desired product and an interesting side product have also been established. Current work has been more focused on determining the effect that substituents have on the course of the reaction.
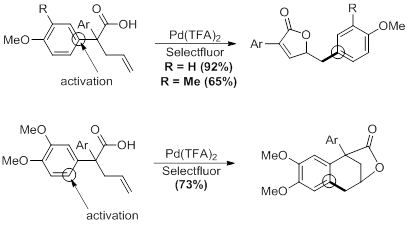
Currently, we are concluding studies into the effect of the activating group’s position on the products formed. The expected unsaturated lactone product is formed in reasonable yields as long as activating groups are present in the para-position of the aromatic ring. Placement of activating substituents in the meta-position resulted in the formation of a tricyclic product through an alternate addition pathway. However, only intractable mixtures are formed when the activating group is placed in the ortho-position. All attempts to optimize the conditions for a single product have failed, and we are currently attempting to isolate and identify the products. We are currently investigating the source of this effect through the placement of nonactivating groups in the ortho- position. We will also explore the effect of the electronic and steric nature of the substituents.
As we are located at a small liberal arts institution, all laboratory was conducted by undergraduate students. Generous ACS-PRF support has enabled three different students to have a fully funded summer research experience, while providing research supplies for an additional four. Students who have worked on this research project intend to enroll in graduate studies in the chemical sciences, seeking both terminal Masters and Ph.D. degrees. Students have gained valuable laboratory experience, learning the technical skills as well as gaining a deeper understanding of chemistry. This research has culminated in a publication in Synthetic Communications in the spring of 2016, numerous poster presentations at local and regional conferences, and an undergraduate poster at the Spring 2016 National American Chemical Society conference in San Diego. In addition to a manuscript in preparation, we have recently published our improved method for the synthesis of the starting diarylacetic acids.