Reports: DNI454435-DNI4: Absolute Stereocontrol of Prochiral Substrates with Chiral Excited State Proton Transfer Dyes
Kenneth Hanson, PhD, Florida State University
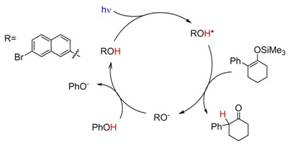
Over the two years the Hanson research group has pursued and achieved our goal of controlling the absolute stereochemistry of organic reactions through a stereoselective excited-state proton transfer (ESPT). During our the first year of this we program demonstrated the photocatalytically generated 2-phenylcyclohexanone from phenyl-2-(trimethylsiloxy)cyclohexene with 7-bromonaphthol (Br-NpOH) as the ESPT catalyst and phenol as the proton source (Figure below).
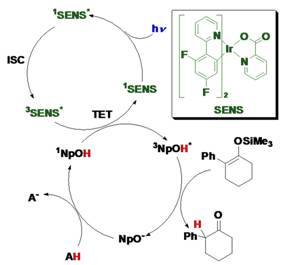
The reaction goes to near completion (96% yield) under a nitrogen atmosphere with only 1 mol % of Br-NpOH as the photocatalyst. Given that the reaction occurs from the triplet excited state of the ESPT dye we also demonstrated that the reaction can also be achieved with visible light (445 nm) upon the addition of triplet sensitizer (Figure below).
The above results were first example of an organic transformation via direct and sensitized ESPT catalysts. These results opened the door to an entirely new class of photocatalytic reactions that harness the acidity of excited state proton transfer dyes. This non-stereoselective ESPT reaction was published in Chemical Communications (Chem. Commun., 2016, 52, 1350-1353) and was highlighted on the back cover. Anjan Das, who is the first author on the manuscript, presented these results at the Gordon Research Conference on Photochemistry and the I-APS meeting and his poster received considerable attention at both meetings.
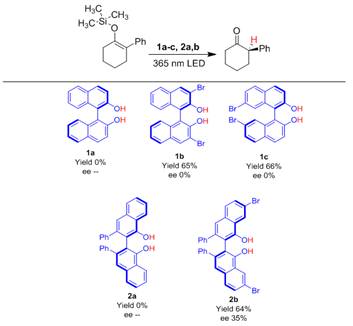
Although the above results were important, the achiral reaction conditions generates a reacemic mixture of (R)- and (S)-2-phenylcyclohexanone as the product. Since the ultimate goal of this work is to achieve absolute stereocontrol of prochiral substrates we shifted our attention to using chiral ESPT dyes for the enantioselective protonation of prochiral enol ether. BINOL and VANOL compounds (structures below) were selected for this work because 1) they contain naphthol chromophoric units which are known to exhibit ESPT and 2) they are relatively common molecules for use in enantioselective reactions because of their axial chirality.
The yield and enantiomeric excess reported in the above figure indicate that both bromine atoms and a sterically hindered dye structure of 2b are needed to achieve an enantioselective reaction in toluene under 365 nm irradiation. The former is to generate a reactive long lived triplet state for the reaction to proceed and the latter is to inhibit planarization and racemization of the dye in the excited state. In the absence of light, 2b is not sufficiently acidic to protonate the substrate and generate any observable product even at elevated temperatures. However, under 365 nm irradiation, with 2b as the ESPT dye, a 35% ee with a 64% yield of product was observed at room temperature. As far as we know this is the first demonstration of enantiopreferential protonation of a prochiral substrates via chiral ESPT catalysis.
Enantioselective ESPT was effective with a range of phenyl-2-(triR)cyclohexene compounds where R = methyl, ethyl, isopropyl, and phenyl groups. The highest ee we we have yet observed, 49 %, was achieved with triphenylsilyl group. This result demonstrates that the asymmetric ESPT reaction offers a simple and attractive route for the generation of optically active carbonyl compounds.
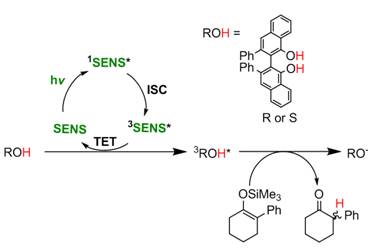
In accord with our previous work, enantioselective ESPT can also be achieved under visible light by using the appropriate triplet sensitizer molecule and the unsubstituted ESPT dye (figure below). Irradiating a mixture of substrate, unbrominated VANOL (2a), and sensitizer with 445 nm light gave (R)-2-phenylcyclohexanone in 68% yield and 25% ee. The same reaction with (S)-VANOL yielded (S)-2-phenylcyclohexanone in similar yield and ee.
In summary we have introduced a new strategy to enantioselectively protonate prochiral substrates using excited state proton transfer from a chiral dye. The reaction is effective with a range of silyl enol ethers and can also be achieved with visible light upon the addition of triplet sensitizer. A manuscript containing these results has been submitted and is currently under review for publication in the Organic Letters. The PI, recently presented these results at the ISPF2 conference in Gotherberg, Sweden where the attendees were particularly interested in our work using sensitizers and triplet energy transfer to drive the reaction.
Despite being very promising, the low ee of the above reaction is due to the racemization of the ESPT dye in the excited state as indicated by circular dichroism. Beyond the current grant, our ongoing effort is to generate new chiral dyes that lack the rotational axis that is present in both BINOL and VANOL. In the absence of racemization we anticipate that we can significantly increase the reaction yield and ee and then expand our substrate scope to include the synthesis of useful fine chemicals like Ibuprofen and others. These promising results have helped the PI to recruit a new graduate student, Suli Ayad, and undergraduate student, Victoria Posey, to pursue this project. They have been working with Anjan to obtain the above results and to generate new ESPT dyes. Additionally, this grant has provided us with the necessary preliminary results for the PI to apply for, and have a strong chance at obtaining, NSF funding next summer (NSF-CAREER) and fall (NSF-CHE).