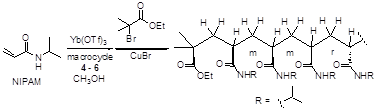Reports: ND754177-ND7: New Catalysts for Stereoselective Polymerization of Functional Alpha-Olefins
Lin Pu, University of Virginia
The research progress during the year of 2015-2016 is summarized below.
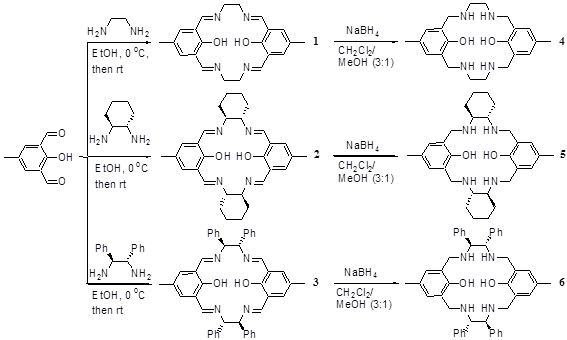
1. Synthesis of the macrocyclic ligands 1 - 6
Scheme 1 shows our syntheses of the macrocyclic imines 1 – 3. In addition, these compounds were treated with NaBH4 in a mixed solvent of CH2Cl2 and MeOH (3:1) to generate the corresponding reduction products, the macrocyclic amines 4 – 6.
Scheme 1. Synthesis of Macrocyclic Imines and Macrocyclic Amines
2. Polymerization of N-isopropyl acrylamide (NIPAM) using the macrocycle-based catalysts
Because poly(N-isopropylacrylamide) [poly(NIPAM)] is a class of very interesting thermo-responsive polymer and has found broad applications in areas such as drug delivery, tissue engineering and biosensing, we explored the synthesis of poly(NIPAM) by using the ATRP method. We tested the polymerization of NIPAM by using the macrocyclic imine 1 (1 equiv) in the presence of CuCl (1 equiv), methyl 2-chloropropionate (1 equiv) and Yb(Otf)3 (5 equiv) in methanol at room temperature. After 12 h, no polymerization was observed. We also tested the polymerization of NIPAM by using the macrocycles 2 and 3 under the same conditions. For the use of 2, only trace amount of the polymer was observed after 12 h at room temperature. Using 3 led to the formation of poly(NIPAM) with an isotacticity of 86%, but the monomer conversion was only about 14% after 12 h at room temperature.
We then examined the use of CuBr and the initiator ethyl 2-bromoisobutyrate in place of CuCl and methyl 2-chloropropionate for the polymerization. We found that the macrocyclic ligand 3 in combination with CuBr, ethyl 2-bromoisobutyrate and Yb(OTf)3 exhibits excellent catalytic activity for the ATRP of NIPAM at room temperature. Under the optimized conditions of [NIPAM]: [Yb(OTf)3]: [CuBr]: [Initiator]: [Ligand] = 100: 5: 2: 2: 1 ([NIPAM] = 3.9 M), poly(NIPAM) was obtained with both high conversion and high isotacticity as shown in Table 1. Compound 3 displays higher catalytic efficiency than 1 and 2.
Table 1. Results for the polymerization of NIPAM by using the macrocyclic imines 1 – 3.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The macrocyclic amines 4 – 6 were also used to catalyze the polymerization of NIPAM under the same conditions as the use of compounds 1 – 3. As the results in Table 2 show, the macrocyclic amines 4 and 6 have exhibited improved catalytic activity and stereoselectivity over their corresponding macrocyclic imines 1 and 3. Among these macrocyclic imine and amine-based new catalysts, compound 6 is identified as the most active as well as the most stereoselective catalyst.
Table 2. Results for the polymerization of NIPAM by using the macrocyclic amines 4 – 6.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We have conducted
kinetic studies for the polymerization of NIPAM catalyzed by the macrocycles 3 and 6. The studies were made
under the optimized polymerization conditions with [NIPAM]: [Yb(Otf)3]:
[CuBr]: [Initiator]: [Ligand] = 100: 5: 2: 2: 1 and [NIPAM] = 3.9 M in methanol
at 20 ℃
![]() . Once the polymerization started, after a
certain time, a small fraction of the reaction mixture was taken out and
exposed to air to quench the reaction, then the conversion of the mixture was
determined by 1H NMR. Figure 1
displays the results obtained for the polymerization catalyzed by using the
macrocyclic imine 3 and Figure 2
shows the results obtained for the polymerization catalyzed by using the
macrocyclic amine 6.
. Once the polymerization started, after a
certain time, a small fraction of the reaction mixture was taken out and
exposed to air to quench the reaction, then the conversion of the mixture was
determined by 1H NMR. Figure 1
displays the results obtained for the polymerization catalyzed by using the
macrocyclic imine 3 and Figure 2
shows the results obtained for the polymerization catalyzed by using the
macrocyclic amine 6.
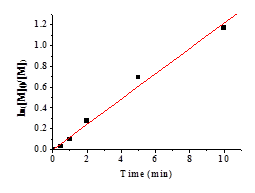
Figure 1b shows a good linear relationship (R2 = 0.9893) between ln([M]o/[M]) and time when the monomer conversion is lower than 70% in the presence of 3. When the conversion is higher than 70%, the more viscous system might slow down the reaction and the relationship is deviated from linear. Figure 1c reveals the relationship between conversion and time. It shows that 70% conversion was reached within 10 min.
Figure 1.
(a) Relationship between ln([M]o/[M])
and time for the polymerization of NIPAM catalyzed by 3. Total ln([M]o/[M])
verse time. (b) Linear region of Figure A (first 10min,
conversion less than 70%, R2 = 0.9893). (c)
Relationship between conversion and time for the polymerization of NIPAM
catalyzed by 3.
(a) (b)
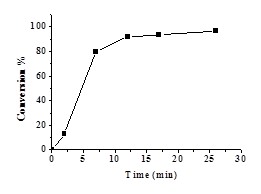
Figure 2a shows the
relationship between ln([M]o/[M]) and time for the polymerization of
NIPAM catalysed by 6. The conversion of the monomer reached 80%
within 7 minutes (Figure 2b), which means the polymerization rate is much
faster by using the macrocyclic amine ligand 6 based catalyst. Overall, this macrocyclic amine catalytic system
is more effective than the corresponding Schiff base-based one. Figure 2. (a)
Relationship between ln([M]o/[M]) and time for the
polymerization of NIPAM catalysed by 6.
(R2 = 0.9211). (b) Relationship between conversion and time for
the polymerization of NIPAM catalyzed by 6.
3. Summary We
have prepared a series of macrocyclic imines and amines and have studied their
use for the ATRP of N-isopropylacrylamide.
In the presence of a Lewis acid complex, these macrocycles have enhanced
catalytic activity over the previously reported catalysts. Good stereocontrol has also been achieved. In the next project period, we will further
modify the polymerization conditions in order to achieve both molecular weight
control as well as stereocontrol.