Reports: UR1053498-UR10: Bottom Up Development of a Gas Hydrate Inhibitor Coating
Paul W. Baures, PhD, Keene State College
ACS PRF Year 3 Report
This project is based on the hypothesis that an inexpensive, durable, and non-toxic polyol-based coating could be developed to inhibit gas hydrate formation in pipelines. This year we gained experimental evidence that a polyol surface was capable of inhibiting nucleation of a 1:15 molar ratio of tetrahydrofuran/water used as a model system of other gas hydrates.
Current Results
A team of three undergraduate researchers (two first-year students and a senior who graduated in December) worked as a team on the project in this past year. Jeffery Hall was the senior and contributed most significantly to the project by working full-time from January-June.
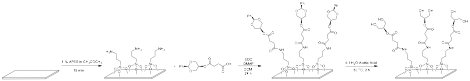
The goal of this year was to test whether the proposed polyol coatings on the inside of glass test tubes offered any inhibition in the nucleation of tetrahydrofuran hydrate. Jeff derivitized glass test tubes as well as microscope slides (Figure 1) to make contact angle measurements as a means of showing support that the surface modification was complete.
Figure 1. Glass derivitization process showing one of the final polyols in this project.
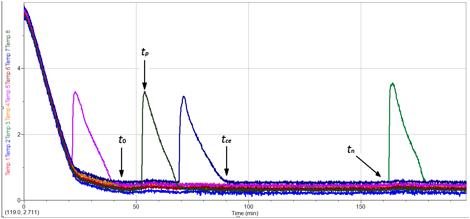
Jeff made an important advance for our research goals by refining how we performed the nucleation experiments. Our earlier efforts were done in a refrigerated water bath that precluded stirring the solutions, and as a result nucleation was limited as compared with the literature model we were following. Jeff plumbed the water bath to an external container that afforded each test tube to be stirred, and as a result we were able to obtain comparable data with previous literature reports for the nucleation experiment (Figure 2).
Figure 2. Experimental setup for the tetrahydrofuran hydrate nucleation experiments.
With the new experimental setup in place, Jeff setup nucleation experiments by adding a solution of 1:15 molar ratio of tetrahydrofuran:water to the test tubes. The solutions were cooled from 5 ¡C to 0.0 ¡C and the nucleation events monitored over the course of 12 hours. The experiments were repeated until a minimum of 50 useable nucleation events were observed, thereby providing enough data to reach meaningful statistical differences. In addition, experiments were done with cleaned glass tubes as one control, as well as with a variety of coatings that were intermediate to the final polyol coatings. Jeff also ran experiments with polyvinylpyrrolidinone that serves as a positive control and is the polymer used by the petroleum industry to prevent gas hydrates in pipelines.
The data obtained from these experiments is illustrated in Figure 2. As the temperature of the bath was lowered to 0.0 ¡C, some solutions would flash freeze. These samples were not included in the final graphs, but instead only those solutions that remained a liquid once the equilibrium temperature was well established were considered. For these solutions, we determined an equilibrium time point (t0) and subsequent times at which nucleation endotherms were observed (tp).
Figure 2. Typical data from the tetrahydrofuran hydrate nucleation experiments.
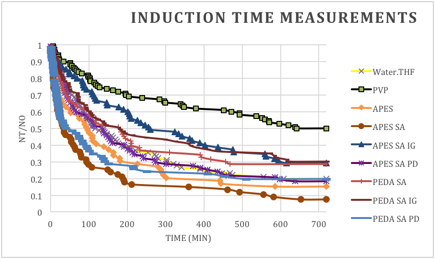
The results of the nucleation experiments are shown in the graph in Figure 3. For the samples that did not flash freeze, 20% of the solutions remained unfrozen after 12 hours (Water.THF). In contrast, the positive control containing PVP had 50% of the solutions remain unfrozen after 12 hours (PVP). The hypothesized polyol coatings to be tested in this project are APES SA IG, APES SA PD, PEDA SA IG, and PEDA SA PD in the graph. The APES and PEDA acronym represent two different silane linkers, the SA represents the succinic acid linker between the silane and the polyol, and the IP and PD represent two different connectivities of glycerol (the polyol) to the linker (the 1,2-diol is the IP acronym and the 1,3-diol is the PD acronym).
The most intriguing of these coatings is the APES SA IG, which resulted in 29% of the samples remaining unfrozen after 12 hours. Likewise, PEDA SA IG had similar percentages remain unfrozen, though more samples froze earlier with this coating. A similar percentage of tubes remained unfrozen in the PEDA SA control. In contrast, a lower percentage, 7.4%, of tubes containing APES SA remained unfrozen. This later coating appears to nucleate crystallization of the hydrate.
The most significant result of this experiment and the most intriguing is the inhibition of nucleation represented by the APES SA IG coating. This surface modification inhibited the nucleation of the THF hydrate early in the time course of the experiment, similar to the control polymer PVP, and proved to be statistically significant as compared to the control tubes (Water.THF). The PVP solution is used at 0.25 mM and our results with this additive are comparable with previous research (Zeng, et al. J. Am. Chem. Soc. 2006, 128, 2844.)
Figure 3. Induction time measurements showing the fraction of solutions remaining unfrozen at different time points.
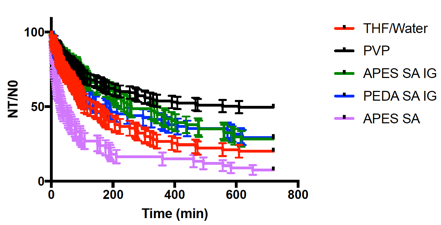
We treated the data to statistical analysis with the method used for generating survival curves for patient populations. The resulting figure and statistical significance for selected samples is shown in Figure 4. This is the first known demonstration of a coating that is capable of inhibiting hydrate formation. Future experiments will investigate a synergy between the coatings and PVP. If it is possible to use a lower concentration of the PVP, this would reflect a cost savings for petroleum companies. Additional polyol coatings will also be investigated in the near future.
Figure 4. Survival curves for selected nucleation experiments.
Impact
The support from this grant has provided real-world research experience for three undergraduates at different stages of their careers. The project has also been of significant benefit to the PI by providing the financial resources to keep undergraduate researchers around during the summer months and pursue this objective. Jeff presented his research at the American Chemical Society Northwest Regional Meeting in Anchorage, AK, in June of 2016. We are planning a manuscript reporting the results for later this year.