Reports: DNI354178-DNI3: Preparation of Low-Coordinate Bis(Alkoxide) Metal Complexes and Their Reactivity in Bond Formation and Bond Activation Reactions Involving Azide Precursors
Stanislav Groysman, Wayne State University
Introduction
This project focuses on the investigation of group transfer reactivity of the low-coordinate base metal complexes in weak-field ligand environments. Toward this goal, we have recently designed a new family of bis(alkoxide) metal complexes M(OR)2(THF)2 (M = Cr – Co) stabilized in a monomeric state by a bulky alkoxide ligand [OCtBu2Ph].1 During the previous funding period, our research focused on the investigation of the nitrene functionality in Fe(OR)2(NAr) complexes.2 In the current funding period, our studies focused on: (1) synthesis and the reactivity of Cr(OR)2(NR1) complexes in nitrene transfer;3 (2) synthesis and characterization of novel high-valent cobalt carbene functionality in weak-field ligand environment.4
Synthesis and Reactivity of Cr(OR)2(NR’) Complexes in Nitrene Transfer
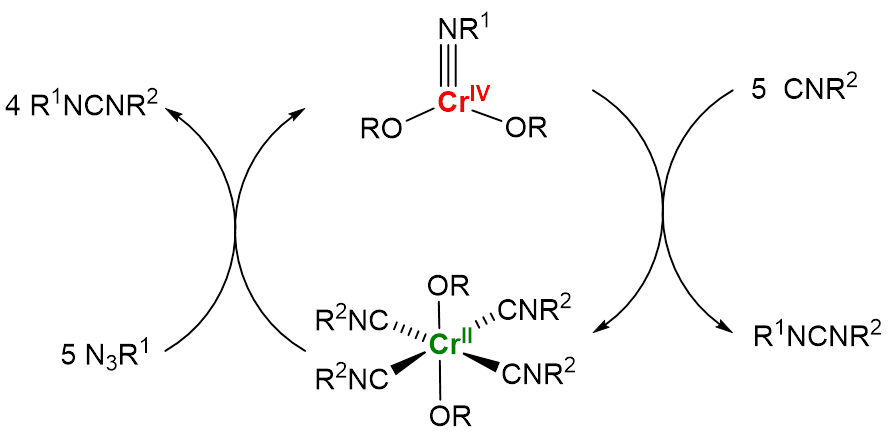
Chromium(IV) imido complexes Cr(OR)(NR1) (R1 = adamantyl, mesityl) were synthesized by the reaction between chromium(II) bis(alkoxide) precursor Cr2(OR)2 and the corresponding bulky azide.3 The complexes were characterized by elemental analysis, X-ray crystallography, solution magnetometry, and IR spectroscopy. Upon treatment with excess 2,6-dimethylphenyl isocyanide (CNR2), both complexes underwent clean reaction to yield the corresponding carbodiimides R1NCNR2 and the chromium(II) complex Cr(OR)2(CNR2)4. Treatment of Cr(OR)2(CNR2)4 with five equivalents of azide leads again to the formation of the corresponding carbodiimide. Based on these results, we postulated that our system may exhibit catalytic reactivity according to the Cr(II)/Cr(IV) cycle presented in Figure 1. To investigate the catalytic performance of the chromium(II) precursor, an equimolar mixture of various azides/isocyanides was subjected to 2.5 mol% of [Cr(OR)2]. These studies revealed that bulky azides (R1 = mesityl, 2,6-diethylphenyl, 2-isopropylphenyl, adamantyl) are capable of formation of carbodiimides in good yields. In a sharp contrast, no carbodiimide formation was observed with smaller aryl azides (R1 = 4-trifuoromethylphenyl and 4-methoxyphenyl). Subsequent mechanistic and theoretical studies indicated that the reaction of smaller azides with chromium(II) precursors formed instead bis(imido) chromium(IV) complexes Cr(OR)2(NR1)2 which were unreactive with isocyanides.
Figure 1. Cr(OR)2-mediated catalytic cycle describing formation of carbodiimides from azides and isocyanides
Synthesis and Characterization of Novel High-valent Cobalt-Carbene Functionality
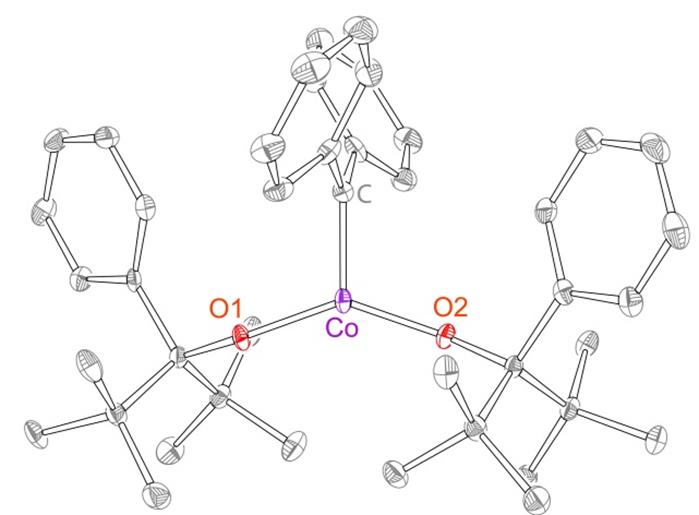
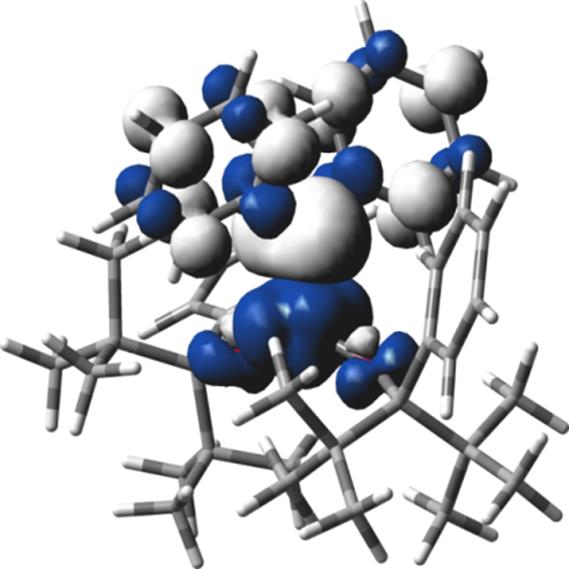
The formation of reactive high-valent nitrene functionalities M(OR)2(=NR1) at iron and chromium centers prompted us to investigate chemistry of the related carbene complexes using the same weak-field bis(alkoxide) ligand scaffold. Treatment of Fe(OR)2(THF)2 with diphenyldiazomethane (N2CPh2, serving as a precursor to diphenylcarbene) led immediately to a color change and N2 evolution. However, the only product that was isolated from this reaction was benzophenone azine Ph2CNNCPh2; its formation could be consistent with a transient carbene species [Fe(OR)2(=CPh2)]. In contrast, treatment of Co(OR)2(THF)2 with N2CPh2 formed stable Co(OR)2(=CPh2) that was isolated in 85% yield. Co(OR)2(=CPh2) was characterized by X-ray crystallography (Figure 2), solution magnetometry (using Evans method), cyclic voltammetry, high-field electron paramagnetic resonance (EPR) spectroscopy and Density Functional Theory (DFT). The solid-state structure demonstrated short Co=C bond of 1.773(3) Å. In contrast, most other Co-carbene complexes found in the literature demonstrate Co-C bonds longer than 1.90 Å. Thus, our structure is consistent with a relatively oxidized metal center and/or strongly bound carbene. Cyclic voltammetry demonstrated three accessible reductions (at −1.17 V, −2.46 V, and −3.16 V), two of which were quasi-reversible; this finding is again consistent with the overall oxidized system. Solution magnetism and EPR spectroscopy both indicated low-spin (S = ½) spin state. The observed g values (gx = 3.04(1), gy = 2.17(1), and gz = 1.91(1)) suggested significant Co-based spin density, as opposed to the mostly carbene-based radical observed in Co-porphyrin systems.5 DFT calculations (using two different functionals) are consistent with the spectroscopic studies, proposing that the lowest-energy spin state of Co(OR)2(=CPh2) is S = ½ and that it features significant CoIV−alkylidene character. Thus, our report describes a new kind of structurally characterized late-metal carbene species that features high-valent carbene functionality.
Figure 2. X-ray structure of Co(OR)2(=CPh2) complex.
Figure 3. Spin density plot for Co(OR)2(=CPh2) (S = ½): α spin is blue and β spin is white.
Summary and Outlook
We have developed a new bis(alkoxide) ligand scaffold that enables formation of low-coordinate nitrene and carbene functionalities M(OR)2(=NR’) and Co(OR)2(=CR’2).6 For Fe, both nitrene and carbene species were highly reactive and could not be isolated, producing the “coupled” products instead, diazene R’NNR’ and azine CR’2NNCR’2. In contrast, stable chromium-nitrene and cobalt-carbene systems were obtained and characterized. Our future studies will focus on further investigation of the iron-nitrene chemistry. We anticipate that the use of bulkier alkoxides may lead to the more stable iron-nitrene functionality. One such alkoxide, [OCtBu2(3,5-Ph2Ph)], has been already synthesized and its reactivity studies are currently underway in our laboratories.
References
1. (a) Bellow, J. A.; Fang, D.; Kovacevic, N.; Martin, P. D.; Shearer, J.; Cisneros, G. A.; Groysman, S. Novel Alkoxide Cluster Topologies Featuring Rare Seesaw Geometry at Transition Metal Centers. Chem. Eur. J. 2013, 19, 12225-12228. (b) Bellow, J. A.; Yousif, M.; Fang, D.; Kratz, E. G.; Cisneros, G. A.; Groysman, S. Synthesis and Reactivity of 3d Metal Complexes with the Bulky Alkoxide Ligand [OCtBu2Ph]. Inorg. Chem. 2015, 54, 5624- 5633.
2. (a) Bellow, J. A.; Martin, P. D.; Lord, R. L.; Groysman, S. Reductive Coupling of Azides Mediated by an Iron(II) Bis(alkoxide) Complex. Inorg. Chem. 2013, 52, 12335 -12337. (b) Bellow, J. A.; Yousif, M.; Cabelof, A. C.; Lord, R. L.; Groysman, S. Reactivity Modes of an Iron Bis(alkoxide) Complex with Aryl Azides: Catalytic Nitrene Coupling vs Formation of Iron(III) Imido Dimers. Organometallics 2015, 34, 2917-2923.
3. Yousif, M.; Tjapkes, D. J.; Lord, R. L.; Groysman, S. Catalytic Formation of Asymmetric Carbodiimides at Mononuclear Chromium (II/IV) Bis(alkoxide) Complexes. Organometallics 2015, 34, 5119 - 5128.
4. Bellow, J. A.; Stoian, S. A.; Van Tol, J.; Ozarowski, A.; Lord, R. L.; Groysman, S. Synthesis and Characterization of a Stable High-Valent Cobalt Carbene Complex. J. Am. Chem. Soc. 2016, 138, 5531-5534.
5. Dzik, W. I.; Xu, X.; Zhang, X. P.; Reek, J. N. H.; de Bruin, B. ‘Carbene Radicals’ in CoII(por)-Catalyzed Olefin Cyclopropanation. J. Am. Chem. Soc. 2010, 132, 10891−10902.
6. Bellow, J. A.; Yousif, M.; Groysman, S. Discrete Complexes of 3d Metals with Monodentate Bulky Alkoxide Ligands and Their Reactivity in Bond Activation and Bond Formation Reactions. Comments Inorg. Chem. 2015, 36, 92-122.