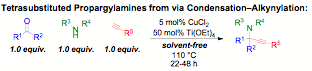58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI151581-DNI1: Catalytic Synthesis of Tetrasubstituted Propargylamines Directly from Simple Starting Materials
Catharine H. Larsen, Ph.D., University of California (Riverside)
As part of a program involving green multicomponentreactions that operate under solvent-free conditions, we have developed methodsfor the synthesis of tetrasubstituted carbons bearing amines. Formed directlyfrom commercially available starting materials, catalytic ketone-amine-alkyne (KA2)couplings have been extended to unactivated, acyclic ketones. Surprisingly,catalytic rather than stoichiometric amounts of Ti(OEt)4 weresufficient to access ketimine, paving the way for the addition of a copper(II)acetylide as the nucleophile. In contrast to the rapid reaction of aldehydes orcyclohexanones in carbonyl-amine-alkyne couplings, it is important to note thatin the absence of the titanium co-catalyst unactivated ketones such as2-butanone provide netiher ketimine intermediate nor propargylamine product. Asovercoming the barrier to ketimine formation is sufficiently difficult torequire dual Cu(II)/Ti(IV) catalysis and 22-48 hours of reaction time, itseemed beneficial to explore other modes of accessing ketimine intermediates.
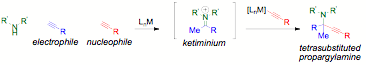
Alkynehydroamination produces ketimines with complete atom economy and should presenta viable alternative to condensation of a ketone and an amine. Generally,intermolecular Markovnikov hydroamination of a terminal alkyne is followed byreduction of ketimine to an amine. In addition, hydroamination has been popularin tandem with hydroarylation for the formation of heterocycles. In over 50 years ofintermolecular hydroamination, the catalytic cycle for the hydroamination of aterminal alkynewith an aminehas not been linked with the catalytic cycle for alkynylation of ketimines to producetetrasubstitutedpropargylamines.
Considerationof these two catalytic cycles immediately shows the problem: an alkyne wouldhave to be activated as an electrophile (to be attacked by an amine to form theenamine that would tautomerize to a ketiminium) and then as the acetylidenucleophile that attacks to form the final tetrasubstituted propargylamineproduct.
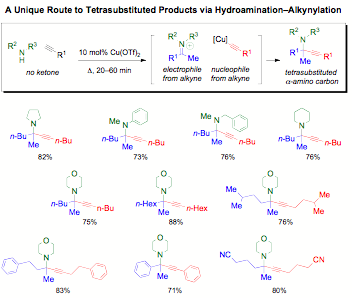
Although copper acetylides arepopular in alkynylation chemistry, of the many metal catalysts for theintermolecular hydroamination of alkynes, copper is rare. In solution, copperforms aldimine from terminal alkynes. However, combining reports on ketimineformation on solid supported copper with the PI's program on solvent-, ligand-,and additive-free catalysis seemed promising. A simple copper salt activates analkyne as an electrophile for Markovnikov hydroamination and as a nucleophileto alkynylate the resultant ketimine in 20 to 60 minutes.
New C–C and C–N bonds are formed by heatinginexpensive Cu(OTf)2 catalyst with an amine and alkyne. Waste is minimized asregioselective hydroamination is achieved in the absence of ligand and solvent.Neither stoichiometric promoters nor co-catalysts are required to aid in thealkynylation of the resultant hindered ketimine intermediates.
The ACS Petroleum Research Fund DNI award was crucial forthese two new catalytic methods to synthesize tetrasubstitutednitrogen-containing compounds directly from commercially available startingmaterials. A rotation of graduate and undergraduate students have broadenedtheir horizons for organic methodology, organic and organometallic catalysts,and mechanistic analysis. In addition to sharing selected portions of thisresearch during the PI's seminar trips, one graduate and one undergraduatestudent have presented at National ACS meetings. In addition to winning threeprestigious UCR science awards, one undergraduate co-author was awarded a BarryM. Goldwater scholarship, an Eli Lilly Women Chemist Committee Travel Award,and a summer internship at Pfizer La Jolla. The results from these past twoyears represent the foundation for a recently-funded NSF CAREER proposal.
Copyright © 2014 American Chemical Society