58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI151029-UNI1: Calcium Catalyzed Homologous Conjugate Addition Reactions
Kristine Nolin, PhD, University of Richmond
Current Results
Development of methodology for the addition of unactivated thiols to donor-acceptor (DA) cyclopropanes has been completed. After a catalyst screen, we found that commerically available calcium acetylacetonate (Ca(acac)2) facilitated the addition of 4-chlorothiophenol, without prior activation or exogenous base, to cyclopropane 1 with conversion of 67% (eq 3). Reaction conditions were optimized and the reaction was probed for generality with regards to substitution on the thiols and the cyclopropane. Electron-rich and electron-deficient thiophenol derivatives as well as alkyl thiols added to provide the corresponding g-sulfanyl malonates in good to excellent yield (Table 1, entries 1-7). The electronic structure of the DA cyclopropane had minimal impact on the productivity of the reaction as good to excellent yields were obtained from the addition of 4-chlorothiophenol to cyclopropanes 2a-h (entries 8-15). This work has been accepted for publication. [1]
We are currently expanding upon our calcium-catalyzed addition reactions by developing catalytic C–C bond forming reactions. Two classes of carbon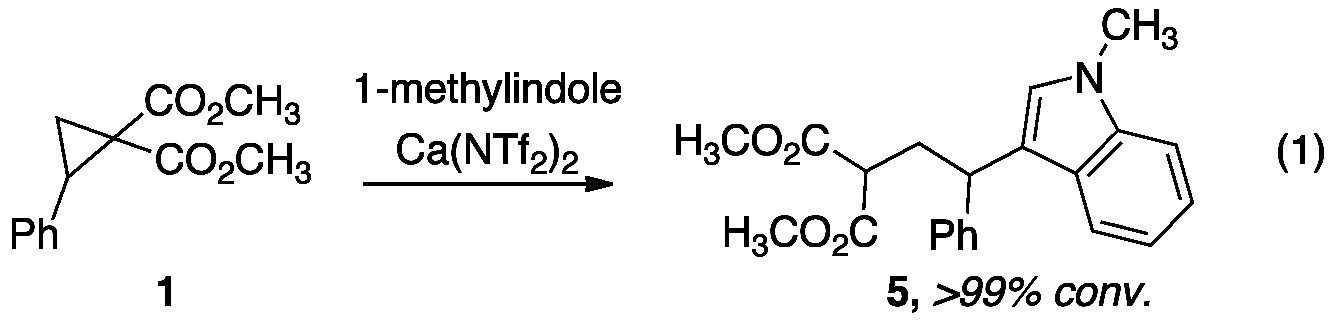 nucleophiles are of particular interest and are being tested as substrates in the HCA reaction: (1) electron-rich aromatic and (2) heteroaromatic compounds. We began our investigation by examining the addition of 1-methylindole to cyclopropane 1 at room temperature in dichloromethane. A screen of commerically available calcium(II) complexes revealed that 10% calcium triflimide (Ca(NTf2)2) facilitated production of a modest amount of HCA product 5. By increasing the temperature to 70 ¡C and switching to 1,2-dichloroethane, the reaction proceeded with >99% conversion as determined by 1H NMR (eq 1). Chlorinated solvents can be avoided by using cyclopentyl methyl ether, CPME, in which the reaction proceeds with 86% conversion. Optimization of the reaction is ongoing. Once the optimal conditions have been established, the scope of the reaction will be examined by varying substitution on the cyclopropanes and indoles.
nucleophiles are of particular interest and are being tested as substrates in the HCA reaction: (1) electron-rich aromatic and (2) heteroaromatic compounds. We began our investigation by examining the addition of 1-methylindole to cyclopropane 1 at room temperature in dichloromethane. A screen of commerically available calcium(II) complexes revealed that 10% calcium triflimide (Ca(NTf2)2) facilitated production of a modest amount of HCA product 5. By increasing the temperature to 70 ¡C and switching to 1,2-dichloroethane, the reaction proceeded with >99% conversion as determined by 1H NMR (eq 1). Chlorinated solvents can be avoided by using cyclopentyl methyl ether, CPME, in which the reaction proceeds with 86% conversion. Optimization of the reaction is ongoing. Once the optimal conditions have been established, the scope of the reaction will be examined by varying substitution on the cyclopropanes and indoles.
 In conjunction with the homologous conjugate addition of indoles, the analogous reaction with benzofuran is being tested. Once again, Ca(NTf2)2 was identified as a robust catalyst for the reaction. Benzofuran added to cyclopropane 1 to provide HCA product 6 in >95% conversion at 90¡C in toluene in the presence of 10 mol% of Ca(NTf2)2 and 10 mol% of Bu4PF6, a co-catalyst (eq 2). This reaction is currently being optimized and will also be explored for generality. In addition to the optimization of the indole and benzofuran addition reactions, we will also be looking at other heteroaromatic compounds as potential nucleophiles, including substituted pyrroles, furans, thiophenes.
In conjunction with the homologous conjugate addition of indoles, the analogous reaction with benzofuran is being tested. Once again, Ca(NTf2)2 was identified as a robust catalyst for the reaction. Benzofuran added to cyclopropane 1 to provide HCA product 6 in >95% conversion at 90¡C in toluene in the presence of 10 mol% of Ca(NTf2)2 and 10 mol% of Bu4PF6, a co-catalyst (eq 2). This reaction is currently being optimized and will also be explored for generality. In addition to the optimization of the indole and benzofuran addition reactions, we will also be looking at other heteroaromatic compounds as potential nucleophiles, including substituted pyrroles, furans, thiophenes.
Impact
During the second year of ACS-PRF support, one research student was supported during summer research (one next year). The student and coworkers made tremendous progress on these projects and were able to gain experience in reaction optimization, synthesis, purification methods, instrumentation usage, and data analysis. Their results were communicated in a publication in Tetrahedron Letters and an award-winning poster presentation at 2012 SERM-ACS meeting.
[1] Braun, C. M.; Shema, A. M.; Dulin, C. C.; Nolin, K. A., ÒHomologous Conjugate Addition of Thiols to Electron Deficient Cyclopropanes Catalyzed By a Calcium(II) ComplexÓ Tetrahedron Lett. (2013), http://dx.doi.org/10.1016/j.tetlet.2013.08.102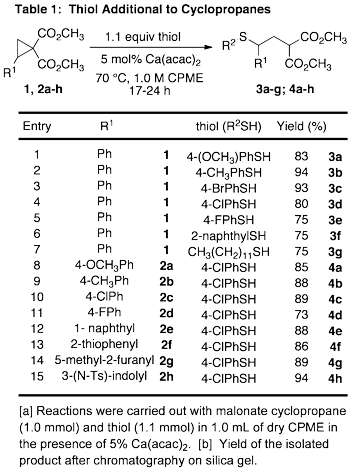
Copyright © 2014 American Chemical Society











