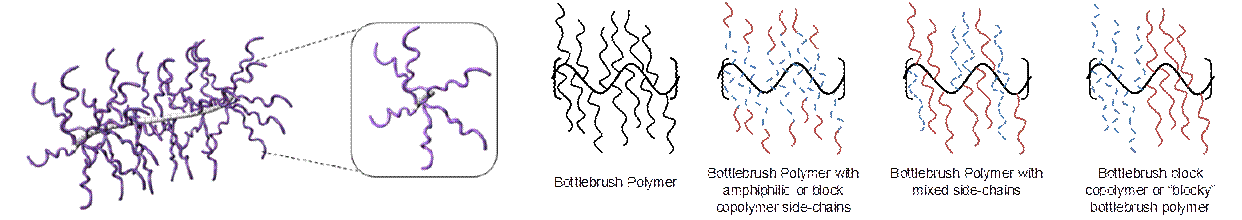58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI752345-DNI7: Elucidating the Structure and Properties of Model Bottlebrush Polymers
Rafael Verduzco, PhD, Rice University
Research into bottlebrush polymers has advanced rapidly over the past 5 years. Bottlebrush polymers were first reported in the 1980s, but the synthetic methods available at the time limited the types of bottlebrush polymers that could be studied. Bottlebrush polymers with broad diversity in molecular structure and composition are now accessible (see Figure 1). However, the size, shape, and conformation of bottlebrush polymers are still poorly understood. While prior work has focused primarily on measuring the length and stiffness of the backbone, the composition and flexibility of the side-chains may be more important for applications that rely on the interfacial properties of bottlebrush polymers.
Figure 1. Schematic for a bottlebrush polymer (left) and for complex bottlebrush polymers with mixed or blocky side-chains (right).
The goal of our research program is to investigate the conformation of bottlebrush polymers in solution and understand the impact that side-chain composition and flexibility may have on interfacial properties, such as contact angles of bottlebrush polymer films or interfacial tension between immiscible liquids. This work may eventually lead to applications in thin film coatings, nanomaterials for drug delivery, and emulsifiers. More broadly, this work has relevance for understanding the interfacial properties of any polymer-coated material, including polymer-coated nanoparticles. In the first year of this project, we studied the thin film properties of bottlebrush polymers with mixed hydrophobic and hydrophilic side-chains, the solution conformation of model polystyrene bottlebrush polymers, and the solubility and interfacial properties of bottlebrush polymers with thermoresponsive poly(N-isopropylacrylamide) (PNIPAAM) side-chains. Each of these projects is described briefly below.
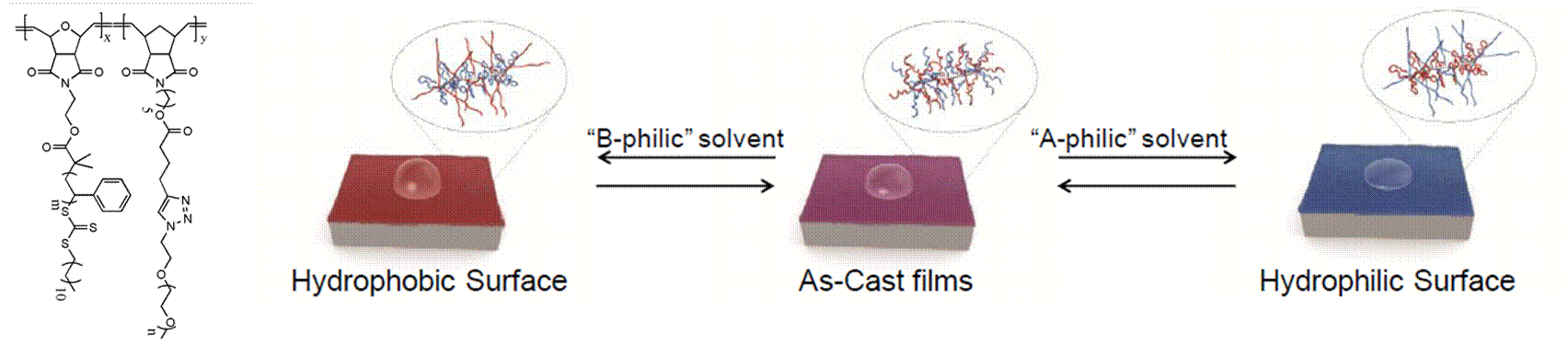
Thin films comprised of end-grafted polymers, known as polymer brushes, have unique properties such as stimuli-responsive surface contact angles and antifouling properties. We hypothesized the bottlebrush polymer thin films would exhibit similar properties due to the high grafting density of side-chains. To investigate this, we prepared bottlebrush polymers with both hydrophobic poly(styrene) (PS) and hydrophilic poly(ethylene glycol) (PEG) side-chains. The relative lengths of the side-chains were varied to study the effect of both relative sizes and flexibility of the chains. Contact angle measurements showed that bottlebrush polymers with longer side-chains exhibited a contact angle that depended on the processing history. Annealing thin films in the presence of hydrophobic or hydrophilic solvents – methanol or hexanes, respectively – resulted in a change in the contact angle as large as 10 degrees. Bottlebrush polymers with shorter side-chains showed a weaker switching response, and bottlebrush polymers with longer PS side-chains exhibited larger contact angles. X-ray photoelectron spectroscopy measurements revealed a change in the relative C and O content near the top film surface, consistent with reorganization of the bottlebrush polymer side chains. This work demonstrates that bottlebrush polymers exhibit stimuli-responsive surface contact angles, similar to that observed for mixed polymer brush films.
Figure 2. Structure of mixed PS and PEG bottlebrush polymer studied and schematic for a stimuli-responsive bottlebrush polymer film.
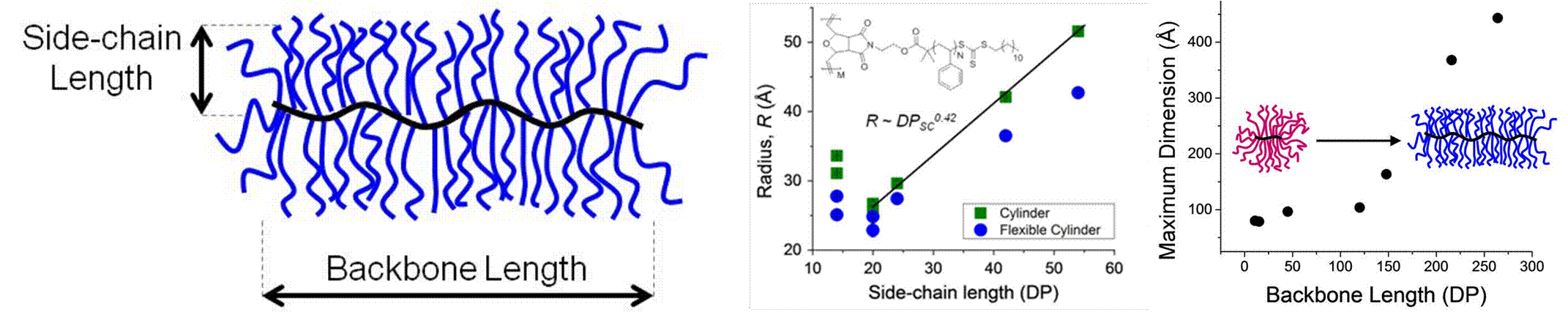
The solution conformation has been previously characterized by small-angle neutron measurements, but prior work focused on a limited set of materials with poorly defined backbone and side-chain lengths in some cases. We focused on quantifying the size and solution conformation of bottlebrush polymers in a model series of bottlebrush polymers made by “grafting-through” polymerization. A series of PS bottlebrush polymers varying in backbone and side-chain length were synthesized and characterized by 1H NMR and static light scattering to obtain absolute molecular weights. Solutions of PS bottlebrush polymers in d8-toluene were analyzed by SANS, and model fitting using a Guinier-Porod and cylinder model revealed that PS bottlebrush polymers with backbone degrees of polymerization lower than 120 exhibited a spherical globule conformation in solution. Above a backbone DP of 120, bottlebrush polymers exhibit an extended, rod-like conformation. This is the first study demonstrating a sphere-to-cylinder transition in bottlebrush polymers.
Figure 3. SANS analysis of PS bottlebrush polymers in d8-toluene including the bottlebrush polymer radius (middle) and maximum dimension (right) as a function of backbone and side-chain DP.
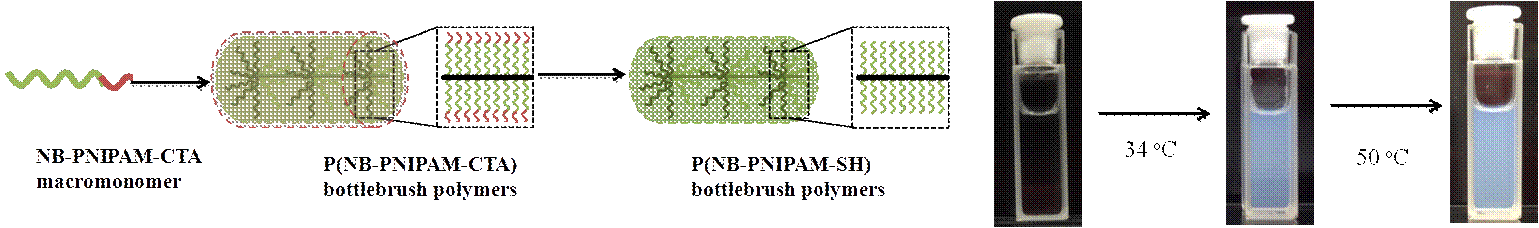
In work recently submitted for publication, we explored bottlebrush polymers with thermoresponsive poly(N-isopropylacrylamide) (PNIPAAM) side-chains. PNIPAAM exhibits a lower critical solution temperature (LCST), and the effect of this transition on densely grafted bottlebrush polymer side-chains is unclear. We hypothesized that a decrease in the solubility of the side-chains may lead to backbone extension. PNIPAAM bottlebrush polymers with side-chain molecular weights ranging from 4 kg/mol – 9 kg/mol were synthesized using a “grafting-through” synthetic approach. Although PNIPAAM is fully water-soluble, bottlebrush polymers were found to be insoluble in water when the side-chains were terminated by a hydrophobic chain-transfer agent (CTA). Furthermore, the presence of a CTA de-stabilized the homogenous phase and decreased the LCST relative to PNIPAAM homopolymer. Removal of the CTA resulted in full solubility and a significant increase in the LCST, from 29°C to 34°C in the case of 4 kg/mol PNIPAAM side-chains. Polarizing optical microscopy measurements revealed a lyotropic liquid crystal phase at temperatures above the LCST for bottlebrush polymers with long PNIPAAM side-chains.
Figure 4. Schematic for PNIPAAM bottlebrush polymers with hydrophobic and hydrophilic side-chains and images of LCST transition in 1 wt % bottlebrush polymer in H2O.
This work reveals new properties and applications of bottlebrush polymers and may lead to their use as thin film additives, emulsifiers, and nanomaterials for drug delivery. The work discussed reflects the efforts of three graduate students (Xianyu Li, Stacy Pesek, and Hadi Shamsijazeyi), two undergraduate students (Sarah Southmayd and Qiqi Xiang), and an assistant professor (Rafael Verduzco). Support from the ACS PRF has been the primary source of support for Xianyu Li and will enable her to complete her thesis work, with a degree in Chemical Engineering expected in May 2014. This work also represents a significant portion of the thesis of Stacy Pesek, expected to graduate with a PhD in May 2015. Undergraduate students have been actively involved, primarily in materials synthesis and characterization. Qiqi Xiang plans to pursue graduate studies in Chemical Engineering after completing her undergraduate studies in May 2014.
Figure 5. Verduzco group, May 2013.
Copyright © 2014 American Chemical Society