58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI752019-DNI7: Bottom-Up Synthesis of Structurally Precise Graphene Nanoribbons
William Dichtel, PhD, Cornell University
1. Synthesis of Dispersible GNRs and Validation of the Benzannulation / Oxidation Approach in Model Compounds
Our project's structurally precise GNRs are derived from a two-step modification of poly(phenylene ethynylene)s (PPEs) that features an unprecedented benzannulation reaction along the PPE backbone, followed by a Scholl oxidation reaction. Our first generation GNR synthesis is shown below (Figure 1). Despite providing promising spectroscopic evidence of GNR formation, this first generation system suffers from extremely poor dispersibility after the final step. This year, we completed an extensive study of model compounds to confirm that the oxidation produces the expected fused structures. These compounds also offer a new means to rapidly access extended and contorted polycyclic aromatic hydrocarbons (PAHs, which have attracted interest for optoelectronic devices.
Figure 1. Synthesis of a structurally precise GNR from a PPE precursor. Each alkyne of the PPE 1 is transformed to a 2,3-disubstitued naphthalene moiety to provide the polyphenylene structure 3. Oxidative dehydrogenation of 3 provides the G1 GNR.
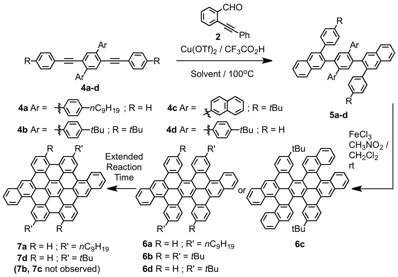
Substituted diarylalkyne derivatives 4a-d are ideal substrates to evaluate the benzannulation/cyclodehydrogenation approach for HBC synthesis (Figure 2). This study confirms that the benzannulation reaction is efficient for several HBC precursors and that typical Scholl oxidation conditions do not induce rearrangements. In some cases, complete cyclodehydrogenation is achieved; however, shorter reaction times or strategically placed substituents provide partially fused products. When complete oxidation does not occur, the order of carbon-carbon bond formation is well defined, providing specific, non-planar coronene derivatives 6a-d that exhibit high solubility. Collectively, these molecular compounds also represent important model systems for oxidizing benzannulated PPEs to produce structurally precise GNRs, and their lack of rearrangements show great promise in this regard.
Figure 2. A series of dialkyne model compounds 4a-4d were benzannulated and oxidized, providing access to a new class of contorted PAHs.
|
Figure 3. A. Rearrangement during the Scholl oxidation observed by King et al. B. Expected and rearranged products for the oxidation of 5b. |
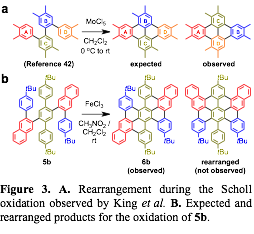
We evaluated the generality and efficiency of the benzannulation reaction across several substituted di(arylethynyl)benzene derivatives. The benzannulation of di(n-nonyl)terphenyl compound 4a corresponds to our G1 GNR system. We also evaluated dialkynes 4b and 4c, which have four and two peripheral t-butyl substituents, respectively. Finally, we evaluated compound 4d, which has 2-naphthyl groups as central aryl substituents. This compound provides access to larger fused aromatic systems and serves as a model for a benzannulated polymer that might provide a GNR (N = 13) with a smooth armchair edge. Dialkynes 4b-4d were each benzannulated efficiently. The G1 model compound 4a was cyclodehydrogenated using FeCl3. Although 1H and 13C NMR spectroscopy of 7a were consistent with the expected structure, the low solubility and propensity of this compound to aggregate prevented definitive structural characterization. Furthermore, King et al. noted rearrangements in a closely related system that had been misassigned in the literature for almost forty years (Figure 3a). Therefore, we prepared several additional model compounds that would exhibit improved solubility to enable unambiguous characterization of the fused PAHs.
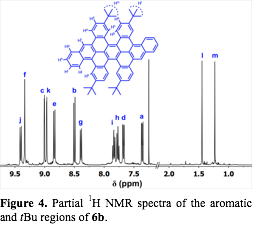
In order to investigate the possibility of rearrangements during the oxidation, we oxidized the compound 5b under the same Scholl reaction conditions. By incorporating the tBu groups on the central terphenyl and external phenyl groups, 5b does not fuse completely to form 6 new C-C bonds. MALDI-TOF MS indicated clean loss of 8 amu, corresponding to the formation of 4 C-C bonds. 1H-NMR of 6b provided unambiguous evidence that 1,2-aryne rearrangements described in the literature do not occur (Figure 4). We hypothesize that steric hindrance associated with these groups causes distortions from planarity that prevent these final two C-C bonds from forming. A derivative that contains central 2-naphthyl substituents (5c), also partially fuses to a similar structure under the oxidation conditions.
|
Figure 4. Partial 1H NMR spectra of the aromatic and tBu regions of 6b. |
The partially fused systems are nonplanar PAHs, as determined by DFT calculations that suggest a ~55 degree dihedral angle between the aromatic rings where the final C-C bond fusion occurs. Contorted HBCs have attracted interest for their shape complementarity to fullerenes and distinct optical and electronic properties. For example, HBCs are typically yellow compounds, whereas solutions of 6a and 6b are orange and those of 6c solution are red. UV/Vis spectroscopy indicated similar spectra for each compound, with optical bandgaps of 2.30 eV for both 6a and 6b and 2.14 eV for 6c. Each compound is strongly fluorescent, and 6a and 6b exhibit identical photoemission spectra with λmax = 505 nm. The emission of 6c has a very similar peak shape and λmax = 540 nm. These maximum emission wavelengths correspond to low Stokes shifts (~25 nm) for each compound that are consistent with their expected rigid structures. Cyclic voltammetry suggested electrochemical bandgaps of 2.01, 2.02, and 1.81 eV for 6a-c, respectively, which correspond well to those determined optically.
This extensive model study demonstrated efficient access to two new classes of PAHs: both fully fused and partially fused hexabenzocoronene derivatives with extended conjugation and differing substitution patterns than those available from existing methods. Most important for graphene nanoribbon synthesis, rearranged products were never observed, independently of whether partially fused or fully fused products are obtained. The partially fused derivatives represent a new class of contorted HBCs that feature high solubility, strong absorptivity in the visible spectrum, and reversible redox processes. The benzannulated derivatives explored here are also important models for accessing GNRs, although complete fusion in the polymer systems must still be demonstrated carefully.
In summary, progress over the last year has involved a significant confirmation and maturation of the benzannulation / oxidation approrach for synthesizing structurally precise GNRs. Challenges to be faced in the upcoming year include improving the dispersibility of the GNRs, identifying methods to deposit and (ideally) align the ribbons on appropriate substrates, and further exploring this reaction as a means to access nanocarbon architectures.
Copyright © 2014 American Chemical Society