58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND350690-ND3: Dimetal Nitrido Chemistry
John Ferguson Berry, University of Wisconsin (Madison)
Scheme 1
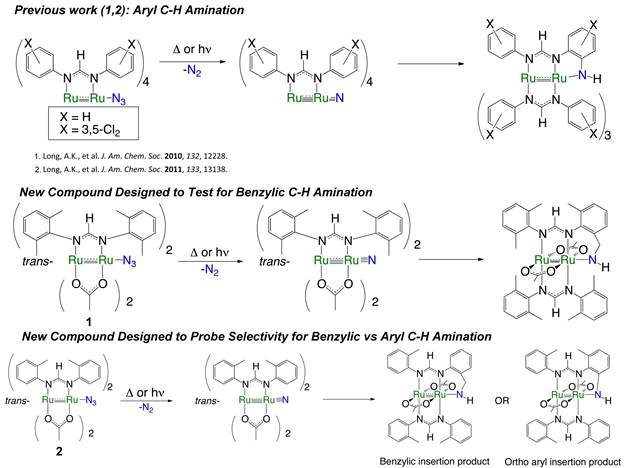
The xylyl substituents on 1 are poised to be attacked by the nitride formed by photolysis or thermolysis of the Ru2-azide group, which can result in the formation of a six-membered chelate ring in the amido product compound. We have monitored this reaction by EPR spectroscopy, which supports the proposed reaction sequence. We hope to be able to confirm the C-H amination product by obtaining a crystal structure.
Compound 2 utilizes o-tolyl instead of xylyl substituents. Thus, once the Ru2-nitride is formed post-photolysis or –thermolysis, the N atom may choose to insert into an aryl or benzylic C–H bond, forming either of the two products shown.
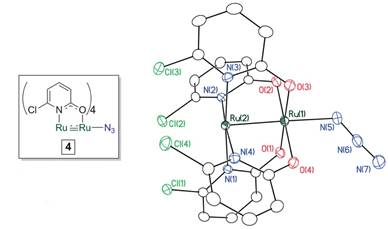
Our next goal was to explore the possibility of intermolecular N atom transfer from Ru2-nitrides. To this end, we have synthesized the Ru2(chp)4N3 molecule (chp = 2-chloro-6-hydroxypyridinate), as shown below in Scheme 2, which features no organic ligand fragments surrounding the azide group due to the arrangement of ligands around the metal. Thus, upon photolytic or thermolytic conversion of the azide to a nitride, no intramolecular C–H functionalization should take place and, instead, it should be possible to observe intermolecular reactivity.
Scheme 2
To date, we have focused on the reaction of the Ru2(chp)4N nitrido intermediate with triphenylphosphine. After photolysis of a solution of Ru2(chp)4N3 in the presence of ten equivalents of PPh3, and following subsequent workup with HCl gas, we have established by 31P NMR that Ph3PNH2Cl is formed in > 60 % yield. This product is proposed to result from reaction of the nitrido intermediate with PPh3 to form a Ru2–N=PPh3 complex, which then reacts with HCl to yield the Ru2(chp)4Cl starting complex (recovered in quantitative yield) and Ph3PNH2Cl. Thus, this reaction represents the first time we have been able to observe intermolecular reactivity with Ru2 nitrido complexes. There are two other noteworthy aspects of this reaction. First, since the Ru2(chp)4Cl starting material is recovered in quantitative yield from this process, we can close a synthetic cycle for converting PPh3 to phosphinimide using stoichiometric amounts of sodium azide as the N atom source (Scheme 3). Moreover, the phosphinimide product, Ph3PNH2Cl, slowly hydrolyzes in water to produce phosphine oxide, Ph3PO, and ammonium ion. Thus, this synthetic cycle may be used to produce ammonia.
Scheme 3
Another question we are currently addressing is whether or not it would be possible to generate the Ru2 nitride complexes from N2. To this end, we are exploring one electron reduction of Ru2(chp)4Cl in attempts to synthesize an N2 complex such as Ru2(chp)4(N2), which would represent the first step towards the goal of converting N2 into a reactive Ru2 nitride. Thus far, we have obtained a number of axial ligand adducts of the reduced Ru2(chp)4 fragment, and have also found that Ru2(chp)4 dimerizes when strongly binding ligands are not present. We have determined equilibrium constants (Keq) for the reaction shown below for a number of different potential axial ligands, L, including N2.
[Ru2(chp)4]2 + 2 L == 2 Ru2(chp)4(L) Keq
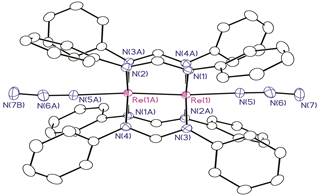
We also have ongoing synthetic efforts to prepare the Os2 and Re2 analogs of the Ru2 nitride complexes described here. We recently reported a preliminary communication of Os2-azide compounds bearing either formamidinate or chp ligands. On the Re2 front, we have prepared an azido complex (Scheme 4) and explored its photolytic and thermolytic properties. The Re2 compounds have the advantage that they are diamagnetic, and thus we can follow the reactions by NMR spectroscopy. It appears that the Re2 compound can undergo conversion to a nitride and intramolecular C–H amination just as in the Ru2 case, but the conversion is at present not good. We are currently working on ways to improve this.
Scheme 4
Copyright © 2014 American Chemical Society