58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND651045-ND6: Energetics and Dynamics of Biofuel Reactive Intermediates
Robert Continetti, PhD, University of California (San Diego)
Energetics and dynamics of biofuel reactive intermediates
This research program has been dedicated to the establishment of the experimental infrastructure for the characterization of the energetics and dynamics of biofuel combustion reactive intermediates. This research program has been motivated by challenges in the use of fuels derived from biogenic sources pose in existing internal combustion engines. Accurate data on the isolated molecules that are present in biofuels is required for first-principles modeling of their combustion processes. In particular, little gas phase data exists for molecules of the size of species like the ester methyl linoleate (C19H32O2) that typically form major fractions of biodiesel fuels, much less reactive radicals derived from these parent molecules. The goal of this project is to provide new information on this class of molecules, including the heats of formation, electron affinities and dissociation dynamics of the reactive radicals produced by hydrogen abstraction from the parent molecules. The experiments will involve negative ion photoelectron spectroscopy and photoelectron-photofragment coincidence (PPC) spectroscopy, made possible for this range of larger molecules by using an electrospray ionization (ESI) ion source coupled with a hexapole ion trap for ion accumulation and a linear accelerator (LINAC) for formation of a 40 keV pulsed ion beam, enabling detection of even large neutral molecules using a microchannel-plate-based particle detector. The experimental efforts are being guided by DFT calculations on the structure and energetics of these species.
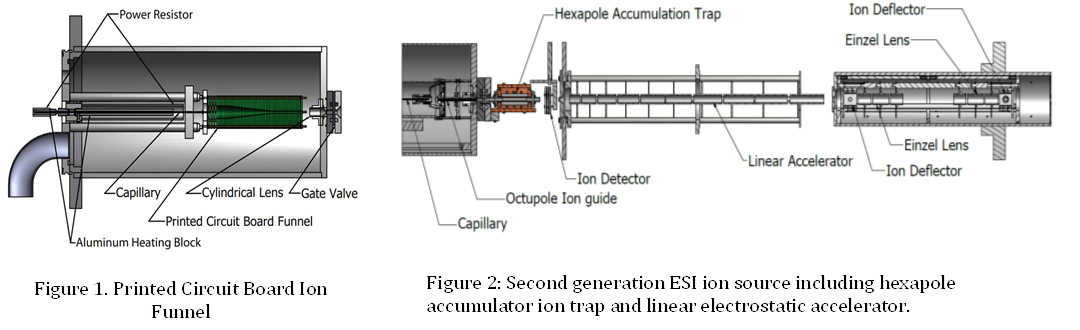
The first year of this grant was devoted to the design, construction and testing of the new ESI source and a novel printed-circuit-board (PCB) based ion funnel, as shown in Figure 1. While the ion funnel was able to produce ion currents of ~1 pA, it was not possible to increase the brightness of this source owing to issues with the pressure in the ion funnel region and limitations in the performance of the PCB construction technique. Therefore, in the second year of the grant a new approach was taken to couple the ESI source with our unique PPC spectrometer. The redesigned source is based on more conventional configurations for ESI sources. Upon exiting the desolvation capillary, the ions now pass through a skimmer, followed by an octopole ion guide, a second skimmer, and then enter a hexapole accumulator ion trap. Experiments have shown promise for this new configuration, with ~5 pA ion current measured at the entrance to the hexapole accumulator trap. To increase the detection efficiency for neutral products of relatively large mass (> 200 amu) we have also implemented an electrostatic LINAC that will allow an increase in the maximum ion beam energy from 10 keV to 40 keV. The new configuration of the ion source as well as the LINAC and post acceleration electrostatic ion optics is shown in Figure 2. At this point, final testing of the apparatus should be completed in the first quarter of 2014, and we will be prepared to study the photodetachment dynamics of the closed shell anions formed by proton abstraction from the biofuel precursor molecules.
 Quantum chemistry calculations were also carried out to complement
the experimental program. Owing to the size of the target molecules, density functional theory (B3LYP/6311+G(d,p)) calculations on the
structure and energetics of both anionic and neutral systems were carried out.
Focusing on the stable anions produced by the different proton abstraction
channels from a series of esters such as methy linoleate and fatty acids such
as oleic acid, as well as the corresponding radicals formed by photodetachment
of the excess electron, adiabatic and vertical electron affinities were
calculated. As often seen in series of organic species, characteristic
adiabatic electron affinities (AEA's) were found for similar anionic moieties.
For example, acyl radical AEA's were found to be ∼1.8 eV, while the
saturated carboxylate radicals exhibited AEA's ∼3.3 eV. The 18 carbon
singly unsaturated oleate carboxyl radical and the 18 carbon doubly unsaturated
linoleate carboxyl radical in particular are predicted to exhibit large
(∼1 eV) differences between the vertical and adiabatic electron
affinities, and thus eliminate carbon dioxide when produced by photodetachment
of the corresponding anions. Based on these findings, the
photoelectron and PPC spectroscopy experiments on these biofuel-relevant
species should provide important and fundamental information on the energetics
and dynamics of this class of molecules. We look forward to acquiring the
experimental data on these systems in the near future and hope to obtain
further research support from the Department of Energy.
Quantum chemistry calculations were also carried out to complement
the experimental program. Owing to the size of the target molecules, density functional theory (B3LYP/6311+G(d,p)) calculations on the
structure and energetics of both anionic and neutral systems were carried out.
Focusing on the stable anions produced by the different proton abstraction
channels from a series of esters such as methy linoleate and fatty acids such
as oleic acid, as well as the corresponding radicals formed by photodetachment
of the excess electron, adiabatic and vertical electron affinities were
calculated. As often seen in series of organic species, characteristic
adiabatic electron affinities (AEA's) were found for similar anionic moieties.
For example, acyl radical AEA's were found to be ∼1.8 eV, while the
saturated carboxylate radicals exhibited AEA's ∼3.3 eV. The 18 carbon
singly unsaturated oleate carboxyl radical and the 18 carbon doubly unsaturated
linoleate carboxyl radical in particular are predicted to exhibit large
(∼1 eV) differences between the vertical and adiabatic electron
affinities, and thus eliminate carbon dioxide when produced by photodetachment
of the corresponding anions. Based on these findings, the
photoelectron and PPC spectroscopy experiments on these biofuel-relevant
species should provide important and fundamental information on the energetics
and dynamics of this class of molecules. We look forward to acquiring the
experimental data on these systems in the near future and hope to obtain
further research support from the Department of Energy.
Copyright © 2014 American Chemical Society











