58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR751997-UR7: Photochemical Control of Nanoparticle Aggregation
William J. Brittain, PhD, Texas State University
· study the SP-MC mechanism using advanced analytical techniques to better interpret kinetics
· synthesize and characterize SP-inspired molecular systems that overcome limitations
·
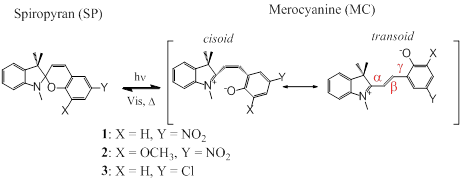
The photoinduced and thermal isomerization of SP have been extensively
studied. The first step in the photochemical process is Cspiro-O
bond cleavage to generate an excited triplet or singlet state that decays
within picoseconds and produces a mixture of geometric isomers of MC that
differ in cis/trans (C or T) conformations about the a, b
and g bonds linking the indole and chromene
subunits (Scheme 2). For
1',3',3'-trimethyl-6-nitrospiro[chromene-2,2'-indoline] (6-nitro-BIPS, SP-1),
the photochemical reaction proceeds via a triplet mechanism followed by
intersystem crossing to 3CCC merocyanine and subsequent conversion
to 3CTC and 3TTC. Theoretical investigations suggest 3CCC*
decays in picoseconds while the lifetime of the 3CTC and 3TTC
species is milliseconds. Evidence for the CTC form of 6-nitro-BIPS
comes from laser-desorption/electron diffraction and excited state dynamics. Here we used electrospray ionization (ESI) ion mobility-mass
spectrometry (IM-MS) to examine both equilibrated and irradiated samples of
spiropyrans 1-3 in methanol (Scheme 2) to gain further experimental
insight into the photoisomerization. IM-MS provides information on the molecules' shapes and
sizes based on collision cross-section (CCS), in addition to mass and
compositional information. Our
interpretation of the IM-MS experimental data argues for presence of a
long-lived (>milliseconds) CCX (refers to two of the four MC isomers with cis
configuration at the central bond) isomer of MC. The lifetime of this proposed
species is considerably longer than the sub-nanosecond lifetimes reported
previously. Scheme 2. General Structures of SPs 1-3 and
their corresponding MCs. Based on our
results, we conclude that the likely reaction pathway is: SP ⇋ CCT/CCC ⇋ TTC/CTC. Theoretical values for the
relative energies of the TTC and TTT isomers revealed a minor difference for
calculations in vacuo versus DCM solution. We feel our
results are reflective of SP-MC dynamics and the contribution of field effects
does not alter our conclusions significantly. Mechanistic elucidation of the
elementary steps in SP-MC isomerization has been largely limited to transient
spectroscopy and model systems. The combination of ESI with IM-MS provides
additional information on the structure-dynamics of this important photochromic
system. This study has
been recently published in Chemical Communications.[3] References 1. “Reactivity of tetrahydrochromeno[2,3-b]indoles: chromic
indicators of cyanide,” Douglas, N.; Neef, C. J.; Rogers, R. A.; Stanley, J.
A.; Armitage, J.; Martin, B.; Hudnall, T. W.; Brittain, W. J. J. Phys. Org.
Chem. 2013, 688-695. 2. “Transient absorption studies of tetrahydrochromeno[2,3-b]indole
ring-opening,” Perry, J. W.; Brittain, W. J. J. Phys. Org. Chem. to be
submitted. 3. A study of the spiropyran-merocyanine system using ion
mobility-mass spectrometry: experimental support for the cisoid conformation,”
Rogers, R. A.; Rodier, A. R.; Stanley, J. A.; Douglas, N. A.; Li, X.; Brittain,
W. J. Chem. Commun. DOI: 10.1039/c3cc47697a.
Copyright © 2014 American Chemical Society