58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI151442-DNI1: Design and Development of Heterocycloaddition Reactions of Aza-Oxyallylcationic Intermediates for Chemical Synthesis
Christopher S. Jeffrey, University of Nevada (Reno)
Since 2010 we have initiated a program that works toward the development of new methods and strategies for organic synthesis based upon the reactivity of electrophilic nitrogen species. Generally, this work was inspired by a biogenic hypothesis that links the diversity of the lyocpodium alkaloids to a common precursor. This hypothesis led us to consider the generation and reactivity of aza-allylic cations. Our early work has focused on the aza-oxyallylic cation, a previously proposed but unknown intermediate in organic synthesis.
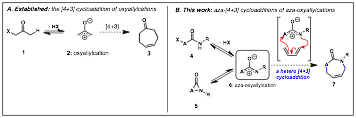
Our group's proposal to intercept the aza-oxyallyl cation 6 in a cycloaddition reaction with dienes was largely inspired related to the work on oxyallyl cations 2 and their use in (4+3)-cycloaddition reactions with dienes to form seven-membered carbocycles 3. With the larger goal of developing new C-N bond-forming reaction methodology, we envisioned that the aza-oxyallyl cation 6 could be similarly employed to create seven-membered heterocycles 7 containing nitrogen and lead to development of alternative approaches to C-N bond formation.
Scheme 1.
Our first focused on the dehydrohalogenation of a-halo O-alkylhydroxamates 4 (X = halogen, R = O-alkyl). In these studies we found that aza-oxyallylic cations can be generated in-situ using basic trifluoroethanol (TFE) or hexafluroroisopropanol (HFIP) and that they react with cyclic dienes to afford the caprolactam 7 in good yield and diastereoselectivity. This was the first example that established definitive evidence of an aza-oxyallylic cation 6. Computational modeling of the uni-molecular ring opening of a-lactams 5 (R1 = Et vs. R1 = OMe) verified the stabilizing role of the N-alkoxy group. These results were published in March 2011 as a communication in JACS. We have extended this concept to the development of intramolecular cycloaddition reactions and the regioselective 1,4-diamination reaction of oxidized urea derivatives. We have begun to pursue alternative methods of generating this intermediate as well as applying these reactions to the synthesis of biologically active target molecules. Recently, our methods have been applied to the synthesis of a new class of degradable polymer that is proposed to have biomedical applications.
Copyright © 2014 American Chemical Society