58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND952025-ND9: Activation of Chemical Reactions Through Corona Discharge
Alexandre Yokochi, PhD, Oregon State University
The objective of this project is to demonstrate that chemical reactions resulting in the partial or full oxidation of organic molecules to effect beneficial transformations can be accomplished using a low power electric corona discharge (i.e., a non-thermal plasma). We propose that successful realization of this goal will enable development of processes like C1 chemistry of methane through CH activation without having to convert the hydrocarbon into synthesis gas, or the oxidative desulfurization of fuels by kinetically fast oxidation of dibenzothiophenes by dissolved oxygen. Potential benefits derived from processes like methane activation include easier routes to convert natural gas to chemicals, or to efficiently capture stranded gas by converting it to useful products rather than flaring it (e.g., associated gas from petrochemical production or anaerobic digestion gas). The efforts from this grant focus on fully characterizing the corona discharge through gases and condensed fluids, initial characterization of the products from the reactions, and initial modeling of the reactions taking place (constructing a 1-D or 2-D model, depending on requirements imposed by the process). Other activities related to this concept such as gas phase CO2 reduction or advanced oxidation of chemical contaminants in water are also interesting and significant challenges, but are beyond the scope of the current study.
To date our efforts have focused on the design, construction and characterization of flow through reactors embodying the corona discharge in both gases and fluids. Others have previously demonstrated that high voltage arc discharge through water or other fluids using applied potentials in the order of 15 kV leads to oxidation of dissolved species. Noting a recent Science article by de Heer showing that by decorating an electrode with carbon nanotubes a sustained plasma could be ignited at lower voltages we hypothesized that more efficient corona discharge activated chemical reactions can be implemented in microchemical systems employing electrodes decorated with CNTs, in which the lower voltages required for sustained corona discharge decreases power consumption and simplifies equipment requirements.
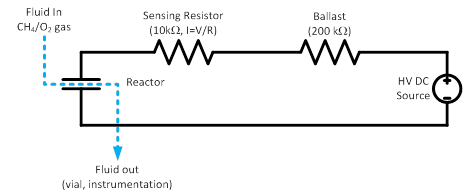
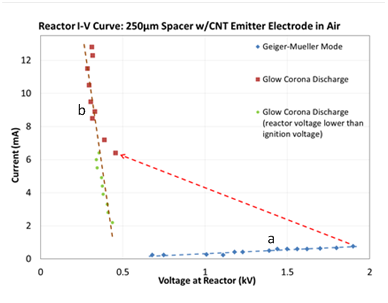
Our first efforts have involved construction of the flow through reactor, assembly of the balance of plant enabling the study of the reactions, and system testing. The reactor in the testbed is shown in Figure 1, and a close up of the outlet of the reactor showing light emitted from the arc discharge within the reactor in Figure 2. The relationship between current and voltage observed for a gas reactor with a gap of 250 µm between electrodes is shown in Figure 3. The voltage supplied to the reactor shows a collapse after a sustained glow discharge is reached at about 1700V (collapse indicated by the red dashed arrow). This collapse is due to a change in operational mode from a “Geiger-Müller” Spark Discharge mode (a in Figure 3) in which little current flows through the system and all voltage applied is present at the reactor walls, to a steady state Glow Corona Discharge mode in which a non-thermal plasma significantly fills the reactor space (b in Figure 3). In regime b, the current flow causes a significant portion of the voltage to be dissipated at a ballast reactor. Clearly, in future implementations of the process, a smart power supply equipped with an electronic ballast that automatically decreases supplied voltage after ignition should be used, but acquiring or designing such a power supply is beyond the scope of the present work.
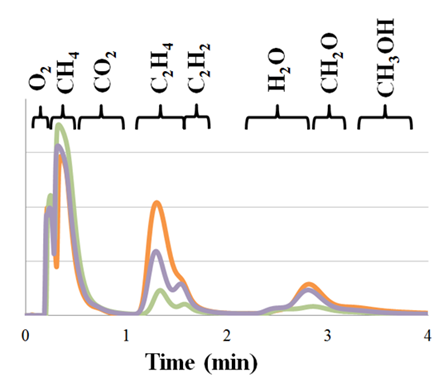
Having developed a viable reactor design and developed a useful testbed in which to evaluate the reactor, attention has turned to characterizing product distributions from the reactor as a function of test conditions (flowrate of gas, gas composition, applied power, temperature, etc). Gas chromatograms of the reactor outlet are shown in Figure 4. The data shown in the orange trace in this figure was produced using a d=750 µm thick reactor in which a 3:1 CH4:O2 gas mixture with a flowrate of V̇=15 sccm at atmospheric pressure and PDC=2.5W (VDC=500V, IDC=5mA) applied to the reactor. The orange trace shows ca. 50% of the CH4 has been consumed, yielding ethylene, acetylene, methanol, and formaldehyde as well as some carbon dioxide and water.
The green trace in Figure 4 was produced using a thinner reactor with the remainder of conditions unchanged (d=250 µm, V̇=15sccm, PDC=2.5W) resulting in shorter residence time of the gas in the reactor and resulting in lower methane conversion. The purple trace in Figure 4 was produced with the thinner reactor but with double the power applied with increased conversion (d=250 µm, V̇=15sccm, PDC=5.0W). Clearly, an interaction between reactor residence time and power applied to the reactor exists in the conversion of CH4 to products. This work formed the basis of three presentations at the 246th ACS national meeting, 2013 AIChE annual meeting and the WCCE9 meetings in which the generous ACS-PRF support for this work was acknowledged.
In the second year of the funding period, work will focus on characterizing reactor performance under different conditions employing both gases and liquids in the reactor, and developing explanatory computational models for the reactor performance.
Figure 1. Diagram of the reaction testbed constructed for this project
Figure 2. Close up view of the energized reactor outlet clearly showing the light emitted from the glow corona discharge within the reactor.
Figure 3. Current-voltage relationship in a 250µm electrode gap reactor showing the voltage applied to the reactor. Two operational regimes can be seen: a-Geiger-Müller Spark Discharge behavior and b-steady state Glow Corona Discharge; the dashed red arrow indicates the collapse of the voltage at the reactor following plasma ignition, with the balance of the voltage dissipated at the ballast resistor.
Figure 4. Gas Chromatogram of the output of a plasma discharge reactors supplied with a 3:1 CH4:O2 mixture at atmospheric pressure. Reactor conditions were: orange: d=750µm, V̇ = 15 mL/min, PDC=2.5W; green: d=250µm, V̇ = 15 mL/min, PDC=2.5W; purple: d=250µm, V̇ = 15 mL/min, PDC=5.0W.
Copyright © 2014 American Chemical Society