58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND451429-ND4: Photochemical Reduction of Carbon Dioxide Using Organic Photocatalysts
Daniel Falvey, University of Maryland
The objective of this project is to identify organocatalysts for the photochemical reduction of carbon dioxide (CO2). Successful realization of this goal will benefit the long-term efforts to mitigate the effects of greenhouse gas emission. However the efforts from this grant focus on identifying methods for harnessing light energy to accomplish initial one or two electron reductions of CO2. In the long term, a useful solution to the problem of CO2 capture and remediation will require coupling the reduction of CO2 to the oxidation of H2O using visible light. This is a formidable challenge and our initial efforts have been focused solely of CO2 photoreduction using electron donors.
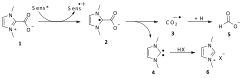
In the previous report we described the use of N-heterocyclic carbenes (NHCs) as photocatalysts. Louie et al. have shown that a variety of NHC's can trap CO2, reversibly forming NHC carboxylate zwitterions (NHC-CO2). It was our hypothesis that the latter species would serve as substrates for photochemical reduction reactions. Our proposed photochemical pathway requires one electron transfer to 1 from an excited state sensitizer (sens*), generating 2 and the cation radical of the sensitizer (sens¥+). Subsequent C—C bond scission of 2 would result in NHC 4 and CO2 anion radical 3. Given the high basicity of 4, it would be expected to protonate in aqueous media yielding imidazolium salt 6. Hydrogen abstraction by radical 3 would lead to formate ion 5 (Scheme 1).
Scheme 1. Proposed photochemical pathway for the production of formate
The initial step in this overall photochemical process was envisioned as an excited state electron transfer reaction from an electron donor to the NHC-CO2 . The thermodynamic driving force (ÆG, in kcal/mol))for this step is determined by the reduction potential (Ered in V) of the acceptor (NHC-CO2), the oxidation potential (Eox) of the donor and the energy available from excitation (Eoo in kcal/mol) as shown in eq 1 (S is a solvation term which is generally negligible in polar solvents). As rule, excited state electron transfer steps have to be exergonic in order to occur with any efficiency. Unfortunately the reduction potentials of the NHC-CO2 species were unknown at the outset of the project.
(1)
Fluorescence quenching experiments are consistent with an electron transfer mechanism as previously described. Six sensitizers with varying Eox* (V) were selected and used in quenching experiments in 1,4-dioxane/H2O solvent mixtures. A representative quenching spectrum and Stern¾Volmer plot is provided (Figure 1). Results show that Eox* of the donor and the rate quenching coincide with each other. N',N',N,N-Tetramethylbenzidine (TMB) exhibits the highest quenching rate whereas 9,10-dibromoanthracene, which is predicted to be the least exergonic, shows negligible quenching results at the concentrations of quencher used (Table 1).
Table 1. Fluorescence quenching results of sensitizers and 1 in 1,4-dioxane/H2O solvent mixtures
Sensitizer | Eox* (V)a | % H2O | kq (M-1s-1) | ΔGET (kcal/mol) b |
TMB | -3.17 | 40 | 3.51 x 109 | -31.6 |
TMB | -3.17 | 50 | 4.67 x 109 | -31.6 |
TMB | -3.17 | 60 | 3.77 x 109 | -31.6 |
TMB | -3.17 | 70 | 2.92 x 109 | -31.6 |
N-Methylcarbazole | -2.46 | 50 | 9.88 x 108 | -17.7 |
2-Aminoanthracene | -2.25 | 50 | 5.36 x 108 | -11.4 |
Anthracene | -2.17 | 50 | 3.30 x 108 | -9.60 |
Phenanthrene | -2.09 | 50 | 1.12 x 107 | -6.36 |
9,10-Dibromoanthracene | -1.89 | 50 | < 107 | -2.08 |
a Eox* = Eox – E00.12
To determine if the photoinduced electron transfer step would result in reductive C—C bond scission, photolysis experiments were conducted at 350 nm using TMB as the sensitizer in solvent mixtures of MeCN/H2O and 1,4-dioxane/H2O (Tables 2 and 3 respectively). Analysis of these mixtures by 1H NMR was used to determine yields of 5 using its resonance at 8.45 ppm. Conversion was determined from the ratio of 1 to 6 and the yields of 5 were based on the assumption that all consumed 1 was converted to 6. Unfortunately, another possible product, oxalate, the dimer of 3, is unable to be detected using this method. Therefore, the latter was analyzed using a colorimetric technique13 and found to be a negligible product
Table 2. Photolysis results of TMB and 1 in MeCN/H2O solvent mixtures
Entry
| TMB [mM]
| 1 [mM]
| Time (min)
| % H2O
| Conversion of 6
| Yield of 5
|
2-1
| 6.9
| 14.7
| 90
| 2.7
| 90.3
| -
|
2-2
| 6.9
| 14.9
| 90
| 5.4
| 100.0
| -
|
2-3
| 10.3
| 14.3
| 90
| 5.4
| 86.0
| 3.9
|
2-4
| 7.3
| 15.8
| 90
| 10.0
| 100.0
| 5.1
|
2-5
| 6.5
| 15.8
| 90
| 15.0
| 93.7
| 4.7
|
2-6
| 2.5
| 14.7
| 90
| 24.3
| 84.5
| 2.4
|
2-7
| 6.4
| 16.4
| 390
| 10.8
| 100.0
| 13.8
|
Table 3. Photolysis results of TMB and 1 in 1,4-dioxane/H2O solvent mixtures
Entry | TMB [mM] | 1 [mM] | Time (min) | % H2O | Conversion of 6 | Yield of 5 | ||||||||
3-1 a | 7.6a | - | 90 | 5.4 | 100.0 | - | ||||||||
3-2 b | 6.6b | 14.5b | 90 | 5.4 | 69.6 | 17.2 | ||||||||
3-3 | 6.2 | 14.9 | 90 | 2.7 | 49.2 | 21.6 | ||||||||
3-4 | 6.5 | 14.5 | 90 | 5.4 | 38.9 | 34.6 | ||||||||
3-9 | 14.1 | 15.0 | 150 | 5.4 | 61.5 | 66.3 | ||||||||
3-10 | 23.1 | 15.6 | 180 | 5.4 | 51.7 | 71.0 | ||||||||
3-11 | 22.6 | 17.6 | 390 | 5.4 | 61.3 | 65.0 | ||||||||
|
|
|
|
|
|
| ||||||||
a Irradiated with 1,3-dimethylimidazolium tetrafluoroborate, 24.2 mM and purged with CO2 in solution for 20 mins.
b Solution heated at 60 °C.
The superiority of 1,4-dioxane over MeCN as the organic component in the binary solvent photolyses leads us to infer that 1,4-dioxane was a superior hydrogen donor to that of MeCN. However, the C¾H bond dissociation energies of the two solvents have comparable (ca. 96.0 kcal/mol) values. In keeping the concentration of TMB the same in photolysis mixtures run in both solvent systems, enhanced formate production in the 1,4-dioxane solutions still remained. Presumably the enhanced production of formate is due solvent polarity effects on compound 1 or intermediate 2. To probe this effect we performed DFT computations at the B3LYP/6-31G(d,p) level using the scrf solvation model on 1 and 2 There appears to be a direct correlation between the solvent polarity and the C¾C bond length between the NHC and CO2. Increasing the polarity of the solvent shortens this bond distance, and presumably strengthens the bond. Thus, we attribute increased yields of formate in 1,4-dioxane/H2O mixtures to the weakening of the binding C¾C bond.
Copyright © 2014 American Chemical Society