58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND551803-ND5: Role of Drainage Channels on Lubrication Forces
Joelle Frechette, Johns Hopkins University
Overview:
The ACS-PRF grant has helped support two separate projects over the last year. The focus of the first project is to understand the adsorption of nanoparticles to the oil-water interface. The goal of the second project was to understand the role of interconnected vertical channels on the fluid pressure necessary to separate two surfaces. Both projects are relevant to the petroleum industry. The ACS-PRF-supported work has led to additional funding from the Office of Naval Research, as well as a long-term collaboration with another faculty. Additionally, the research results have resulted in two published manuscripts.
Summary of Activities:
1) Nanoparticles at interfaces
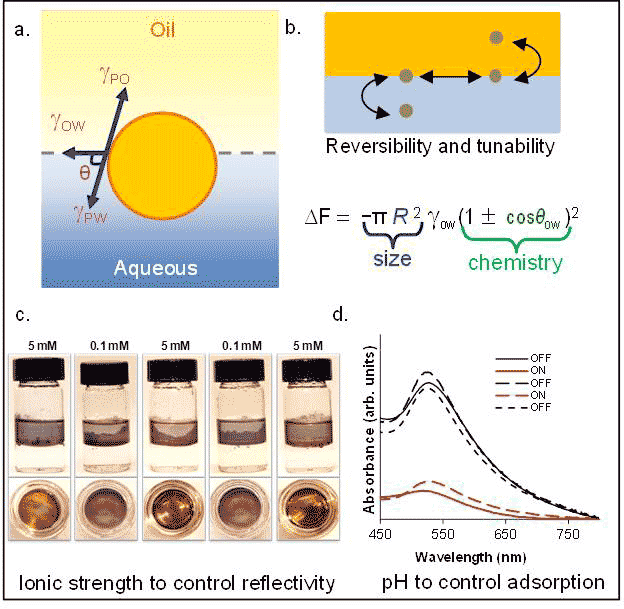
In contrast to the dry configurations, control over the reversible adsorption of nanoparticles at the oil-water interface would allow for the in situ manipulation of the equilibrium array configuration by allowing for particles to leave the fluid interface in a reversible and controllable manner (Fig. 1). Such dynamic control requires a detailed understanding of the competing molecular and nanoparticle interactions. Active control of nanoparticle assembly could contribute to the understanding of particle adsorption in classical problems such as flotation[1], emulsification[2], or transport in unsaturated porous media[3].
Achievements. We demonstrated the mechanisms driving the interfacial adsorption of 5 nm ion-pair functionalized gold nanoparticles to the toluene-water interface.[4] We observed that we could reversibly control the adsorption of the particle to the fluid interface by varying the pH of the aqueous phase (Fig. 1d). By performing a quantitative analysis of the UV-Vis absorption spectra, which we combined with zeta potential, contact angle measurements, and IR spectroscopy, we found that change between the nanoparticles being adsorbed at the interface to being dispersed in the solution could be explained in terms of an interplay of electrostatic repulsion between nanoparticles and the energy change associated with wetting nanoparticles in different media. We also demonstrated that we could control the reflectivity of the interfacial film by varying the ionic strength of the aqueous phase (Fig. 1c). Currently we are working on developing a rigorous thermodynamic model for the reversible nanoparticle adsorption. We also aim to investigate how nanoparticles adsorption is altered by dynamic transport, as what in unsaturated porous media.
Fig. 1. a. Particle at the oil-water interface, b. driving force for particle adsorption at equilibrium. c. Results showing the control over particle spacing at the oil-water interface using ionic strength, d. Results showing the control over adsorption via the change in the pH. |
2) Role of interconnected vertical channels.
Rock masses contain complex and interconnected fracture networks, which are important for oil recovery, groundwater resources, and the generation of power from geothermal sources.[5] Hydrofracture is a practice in which fluid pressure causes the initiation or propagation of a crack in a reservoir. This practice is commonly employed for secondary and tertiary oil recovery where water at high pressure is inserted into a well to increase its productivity. Important fundamental questions relevant to hydraulic fracturing are 1) What is the necessary fluid pressure to initiate or propagate a fracture? 2) What is the timescale for this process? and 3) How to ensure that the fluid injected will not only open the pore space but will also allow for oil recovery in all the interconnected channels forming the porous material?
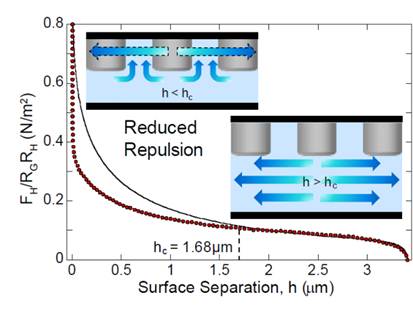
Achievements. We performed direct force measurement of the normal hydrodynamic interactions during the drainage of fluid from the gap between a structured and a smooth surface using the surface force apparatus (SFA). [6] The structured surface consisted of a hexagonal array of cylindrical posts to represent the network of interconnected channels (see Fig. 2). The measured hydrodynamic drainage forces agree with the predictions form the Reynolds' theory for smooth surfaces at large separations. Deviations from theory, characterized by a reduction in the hydrodynamic repulsion, are observed below a critical separation. We employ a scaling analysis to establish the interplay between structural features and the critical separation for the onset of deviations. We estimate a characteristic length scale that corresponds to the transition between fluid being radially squeezed-out of the nominal contact area to fluid being squeezed-out through the network of interconnected channels.
|
Fig. 2. Hydrodynamic drainage forces caused by the presence of a network of channels.[19]. |
[1] a) Langmuir, Trans. Faraday Soc. 1920, 15, 0062; b) Rubio, Miner. Eng. 2002, 15, 139; c) Sutherland, J. Phys. Colloid Chem. 1948, 52, 394.
[2] a) Ashby, Binks, Phys. Chem. Chem. Phys. 2000, 2, 5640; b) Binks, Clint, Langmuir 2002, 18, 1270; c) Binks, Lumsdon, Langmuir 2001, 17, 4540; d) Melle, Langmuir 2005, 21, 2158; e) Pickering, J. Chem. Soc. 1907, 91, 2001; f) Vignati, Langmuir 2003, 19, 6650.
[3] a) Kim, Radiochim. Acta 1991, 52-3, 71; b) DeNovio, Vadose Zone J. 2004, 3, 338; c) Wan, Wilson, Water Resour. Res. 1994, 30, 11; d) Powelson, J. Environ. Qual. 1990, 19, 396.
[4] Luo, Soft Matter 2012, 8, 11923.
[5] Council, Rock fractures and fluid flow: Contemporary understanding and applications, National Academy Press, Washington, DC, 1996.
[6] Gupta, Frechette, Langmuir 2012, 28, 14703.
Copyright © 2014 American Chemical Society