58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI552636-DNI5: Effects of Oxygen Vacancies of Catalyst Surfaces on Reactivity and Selectivity of Carbon Dioxide Reduction with Hydrogen
Franklin (Feng) Tao, PhD, University of Notre Dame
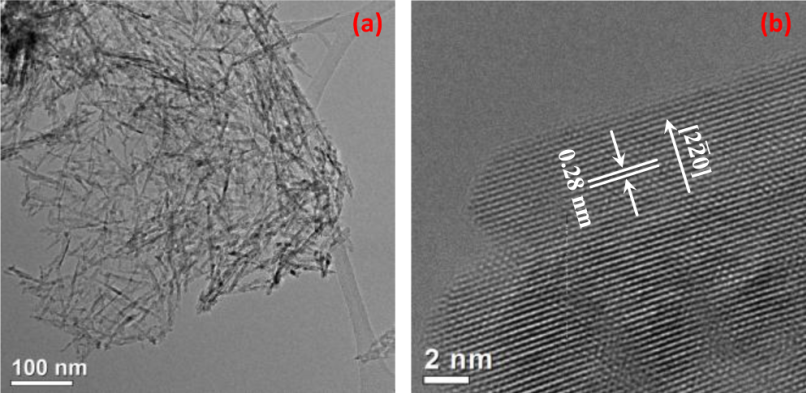
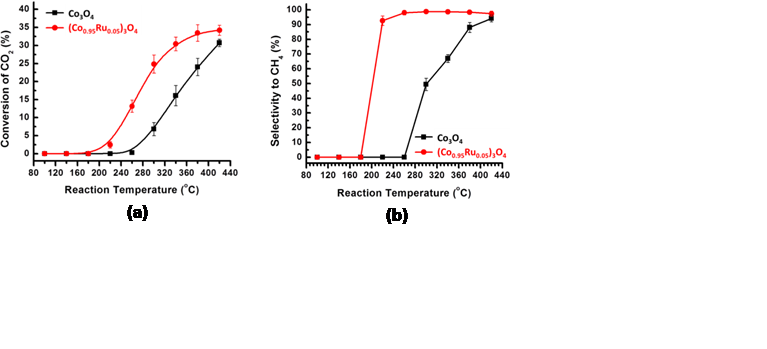
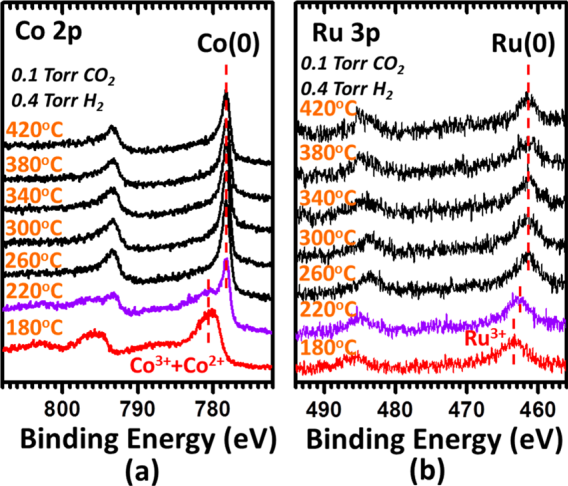
Most transition metal oxides are reducible. Many of them exist in different phases and exhibit multiple oxidation state. Some of them, such as VOx, Fe3O4, and CeO2 are catalysts or active components in heterogeneous catalysis. These reducible oxides play important roles in many catalytic processes. Generally, they exist in three categories: nanoparticles of a doped oxide, a thin film of a doped oxide on an inert support, and nanoparticles of an oxide MOy with immobilized metal or oxide nanoclusters. Our hypothesis was that the surface chemistry (oxidation state, density of surface oxygen vacancies, composition, electronic state, etc) and structure of the doped bulk oxide catalysts during catalysis are quite different from those of doped surfaceoxide. Based on this hypothesis, the surface chemistry and structure of oxide could experience significant change under reaction condition or during catalysis. To understand catalysis on oxide thin film, in-situ studies of surface chemistry is necessary. In the last year (September 2012-August 2013) we have performed operando studies of surface chemistry of that of oxide catalysts and simultaneously measured their catalytic performances for CO2 conversion with H2 to CH4. We prepared Ru-Co mixed oxide and catalytic performance of this catalyst for CO2 reduction with H2. Figure 1 shows the TEM images of the catalysts for CO2 conversion with H2 to CH4. Activity and selectivity in CO2 reduction was measured toward mechanistic understanding the difference of catalytic behaviors between bulk and surface doped oxides. Figures 2a and 2b present the measured catalytic activity and selectivity for conversion of CO2 to CH4. We put emphasis on mechanistic understanding of the difference in catalytic performance between doped bulk oxide and doped surface oxide using the new approach of operando studies. In detailed we have performed operando studies of CO2 reduction with H2 on (Co1-xRux)3O4+y nanorods and thin film by using in-house ambient pressure XPS. Figure 3 is the photoemission features of Co 2p and Ru 3p as a function of catalytic temperatures in the mixture of reactants of CO2 and H2. A correlation between catalytic performances of the two catalysts and their surface chemistry under reaction conditions was built. Active phases of the two catalysts are metallic cobalt and bimetallic Co−Ru, respectively. Light-off temperature of Co−Ru catalyst is lower than that of a cobalt catalyst. Selectivity to production of CH4and activity on the Ru-doped cobalt oxide are obviously enhanced by formation of bimetallic Co−Ru ultrathin film in its surface region in contrast to that of cobalt catalyst in the temperature range of 200−340 °C.
Figure 1. TEM images of synthesized Co3O4nanorods. (a) Largescale image; (b) high-resolution image of a nanorod.
Figure 2. Catalytic conversion (a) and selectivity (b) of CO2 conversion on Co3O4(black line) and (Co0.95Ru0.05)3O4(red line).
Figure 3.Photoemission feature of Co 2p (a) and Ru 3p (b) of (Co0.95Ru0.05)3O4under reaction conditions in the temperature of 180−420 °C. The ambient pressure XPS measurements were performed in the temperature range of 30−420 °C. Data at temperature below 180 °C is the same as those of 180 °C.
Copyright © 2014 American Chemical Society