58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND352242-ND3: Polynuclear Manganese-Oxo Clusters as New Types of Homogeneous Electrocatalysts for Water Oxidation
George Christou, PhD, University of Florida
The grant is supporting an investigation of the ability of molecular high oxidation state Mn/O/carboxylate clusters to function as homogeneous electrocatalysts for water oxidation at low over-potentials. Initial work has been concentrated on the [Mn12O12(O2CR)16(H2O)4] (4MnIV, 8MnIII) clusters, which have been studied for many years for their unusual magnetic properties and for which various modification methods are available. Their attractiveness as potential oxidation catalysts has been recognized for many years, because they contain a cheap 3d metal and simple organic carboxylate groups, comprise high oxidation state MnIII/IV that are strong oxidants, and Mn is the metal of evolutionary choice within the photosynthetic oxygen-evolving Mn4Ca center - their instability to hydrolysis in aqueous solutions has prevented their prior employment for water oxidation catalysis or electrocatalysis. However, we have now developed a means to make them soluble and stable in aqueous solution, and have found them to display promising electrocatalytic water oxidation activity.
Water solubility and stability have been introduced by substituting the 16 acetate groups of
 [Mn12O12(O2CMe)16(H2O)4],
which can readily be prepared in gram quantities, with 3,5-dihydroxybenzoic
acid (dhbH). The many –OH groups impart water solubility and the bulky dhb-
groups protect the Mn/O core of the molecule from hydrolysis. We have carried
out extensive investigation of this compound, and confirmed the reproducibility
of its synthesis and properties. We have also confirmed that it is stable for many
weeks in aqueous solution, with no apparent change to its solution behavior
when studied electrochemically. One question we had was whether it was indeed
possible to put 16 bulky dhb- groups around the Mn12 core,
and addressed this point by making the related compound with (slightly bulkier)
3,5-dimethoxybenzoate (dmb-) (Fig. 1), which does not have –OH
groups, is consequently not hygroscopic like the dhb- cluster, and
thus can be better characterized by elemental analysis. This confirmed that the
product has completely exchanged all the acetates with dmb- groups,
and supports the same situation to have occurred with dhb-. We are
planning a complete characterization of the dmb- derivative by X-ray
crystallography.
[Mn12O12(O2CMe)16(H2O)4],
which can readily be prepared in gram quantities, with 3,5-dihydroxybenzoic
acid (dhbH). The many –OH groups impart water solubility and the bulky dhb-
groups protect the Mn/O core of the molecule from hydrolysis. We have carried
out extensive investigation of this compound, and confirmed the reproducibility
of its synthesis and properties. We have also confirmed that it is stable for many
weeks in aqueous solution, with no apparent change to its solution behavior
when studied electrochemically. One question we had was whether it was indeed
possible to put 16 bulky dhb- groups around the Mn12 core,
and addressed this point by making the related compound with (slightly bulkier)
3,5-dimethoxybenzoate (dmb-) (Fig. 1), which does not have –OH
groups, is consequently not hygroscopic like the dhb- cluster, and
thus can be better characterized by elemental analysis. This confirmed that the
product has completely exchanged all the acetates with dmb- groups,
and supports the same situation to have occurred with dhb-. We are
planning a complete characterization of the dmb- derivative by X-ray
crystallography.
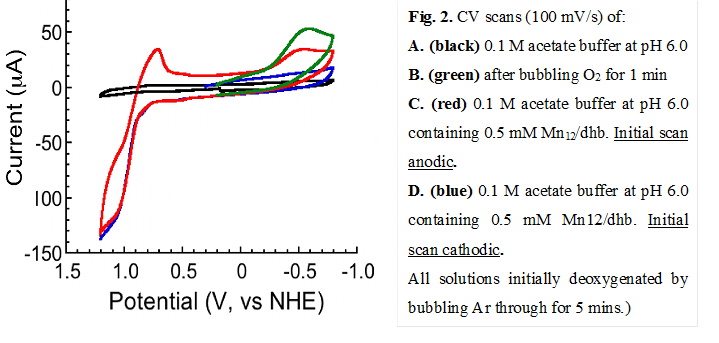
The preliminary electro-catalysis results are very promising and have been reproduced multiple times. Shown in Fig. 2 are the cyclic voltammograms of a 0.5 M solution of the Mn12/dhb- cluster in 0.1 M buffer solution at pH = 6.0. Even after a single anodic scan through the oxidation feature at Ep ≈ 1.08 V vs NHE (Ep is the peak potential), a feature is seen on the return cathodic scan at ~ -0.5 V that is suggestive of the reduction of O2 (red scan C). The feature is absent if the initial scan is anodic (blue scan D), but is seen in a solution of buffer alone through which O2 has been bubbled (green scan B). The conclusion is that oxidation of the Mn12 cluster triggers O2 generation by an as yet unknown mechanism but at a low overpotential of ~ 200 mV.
We have also carried out the CV experiments at different pH values in the pH = 3.0 – 6.0 range, and as expected have found a steady decrease in the oxygen evolution activity with decreasing solution pH.
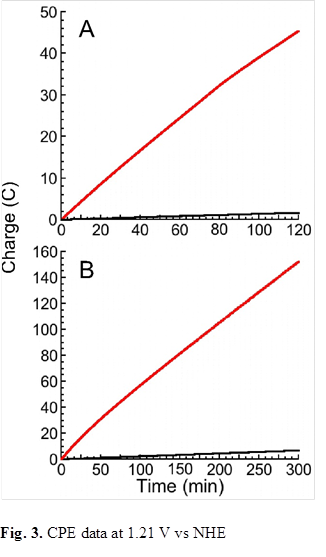
Controlled potential electrolysis (CPE) experiments were carried out in 0.1 M acetate buffer at pH 6.0 in a double-compartment cell at an applied potential of 1.21 V vs. NHE to assess the efficacy of Mn12/dhb as a catalyst. Mn12/dhb is an effective catalyst for the electro-oxidation of
water, with charge accumulation of 45.4 coulombs (C) in 2 hours (A, red line), whereas a control experiment run under identical conditions, but without the catalyst, showed a charge accumulation of only 1.9 C (A, black line). Small gas bubbles were seen to form at the electrode, and these were confirmed as O2 with a NEOFOX oxygen fluorimeter. Similar results were obtained over 5 hours (B, red line), with charge accumulation of 152.4 C (control: 6.9 C, black line). This corresponds to a turnover number (TON) of 59.6 moles of O2 per mole of catalyst, with a turnover frequency (TOF) of ~12 moles of O2 per mole of catalyst per hour.
We are currently extending this investigation to other solubilizing/stabilizing carboxylates, such as the 4-hydroxybenzoic acid (4-hbH in Fig. 1), to assess electrocatalytic activity as a function of ligand type and content of –OH groups, which we believe may be involved in the catalysis as proton shuttles out of the water binding sites during the oxidation.
Copyright © 2014 American Chemical Society