58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051436-DNI10: Synthesis and Structure of Layered Perovskite-Zirconia Thin Film Interfaces: Model Systems for Fuel Cell Studies
Steven M. May, PhD, Drexel University
Motivation: Solid oxide fuel cells (SOFCs) are promising systems for energy conversion due to their high efficiency (and resulting low output of pollutants) in converting a wide range of fuels into electricity. Despite the potential of this technology for efficient energy conversion, a number of materials-related issues, such as unwanted reactions between the cathode and electrolyte, remain as impediments to the greater implementation of SOFCs. The project focuses on the synthesis of perovskite films as model cathode systems and atomically abrupt cathode/electrolyte interfaces, which can provide insight into interfacial reactions in SOFCs. By studying these idealized systems, the project aims to provide guidance into how the performance and interfacial stability of SOFCs can be improved.
Results: Previously in the first year of the project, we synthesized La1-xSrxMnO3 (LSMO) and La1-xSrxFeO3 (LSFO) perovskite films on yttria-stabilized zirconia (YSZ) single crystal (001) substrates using oxide molecular beam epitaxy. These materials are chosen as LSMO and LSFO are commonly used cathodes in SOFCs, while YSZ is the most commonly employed electrolyte in SOFCs. The morphology and atomic structure of these perovskites on YSZ were investigated using atomic force microscopy, synchrotron x-ray diffraction, and transmission electron microscopy. Using these techniques, the epitaxial orientation of the films was determined and the abrupt nature of the perovskite/YSZ interface was confirmed. These results were published in the Journal of the Electrochemical Society.
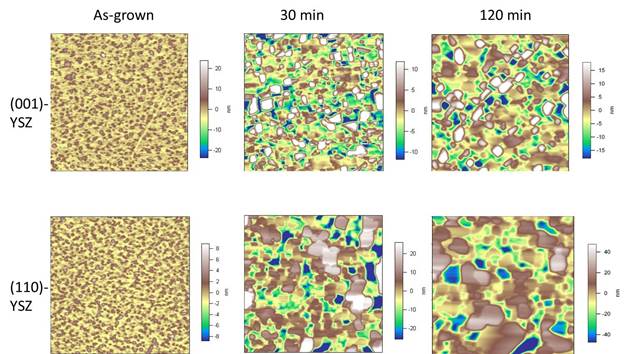
In the past year, we have built upon these results by investigating the perovskite/YSZ interfacial stability against high temperature annealing, in an effort to understand intermixing reactions that can occur at high temperature SOFC operation. We have found a substantial roughening of the film surface at temperatures of 900°C and greater, suggesting this temperature represents the approximate threshold for interfacial reactions. We also find that the magnitude of the roughness depends on the crystallographic orientation of the YSZ substrate, with films grown on (110)-oriented YSZ exhibiting much higher values (~2x) of roughness after 1 hour of annealing than films on (001)- or (111)-oriented YSZ. This result suggests that the interfacial energy, which will depend on the crystallographic orientation, is highest for the (110)-oriented junction, thus enhancing the kinetics of intermixing. Figure 1 shows atomic force microscopy images of as-grown LaMnO3 films on (001)- and (110)-oriented YSZ, and the same films after annealing in air at 1050°C. Note the differences in the height scale bars for the two different films. We have also performed x-ray diffraction on the films after different annealing times in order to identify the composition of any secondary phases that form at the interface. We observe new peaks after annealing at 900 and 1050°C. Analysis of these peaks, which is still underway, suggests the presence of La2Zr2O7, a phase that has previously been identified in bulk SOFC studies.
Figure 1. The evolution of the surface morphology in LaMnO3 films grown on (001)- and (110)-oriented YSZ substrates. |
Building on the success in synthesizing ABO3 perovskites, we have also synthesized A2BO4 layered perovskite films on YSZ. These efforts have focused on nickelate and ferrite, La2NiO4 and LaSrFeO4, films. X-ray diffraction analysis of these films confirms that they are single phase with only a single orientation along the growth direction. As can be seen in Figure 2, the layered perovskite grows with the (110) direction parallel to the YSZ (001), similar to what we observed for ABO3 perovskites on YSZ. Work is currently underway to determine the in-plane epitaxial relationship and to confirm the cation stoichiometry of the film. The next step in this effort will be to investigate the high temperature stability of the layered perovskite/YSZ interfaces and compare to the conventional perovskite/YSZ interfaces.
Figure 2. X-ray diffraction data obtained from a La2NiO4 film on a (001)-oriented YSZ substrate. |
In addition to these efforts, my group has been investigating oxidation and reduction reactions in La0.3Sr0.7FeO3 films. We have discovered that this compound can undergo reversible oxidation/reduction at 200°C, with oxygen loss occurring in air and reoxidation occurring in a mixed O3/O2 environment. This temperature is considerably lower than previous reports of oxygen storage materials, which typical undergo oxygen loss at ~400°C. We have studied the oxygen loss and uptake using x-ray diffraction, resistivity, and optical spectroscopy measurement, revealing that the oxygen loss leads to a significant lattice expansion, a 105 fold increase in resistivity, and the opening of a ~2 eV band gap. Figure 3 shows these changes induced by oxygen loss and uptake. These functional properties can all be reversed back to their original values by reoxidizing the film. The very low temperature at which these reactions occur suggest that this composition of LSFO has excellent oxygen reduction activity and a very large ionic conductivity. These results have been accepted for publication in Advanced Materials.
Figure 3. Electrical resistivity (a) in a La0.3Sr0.7FeO3 film as a function of heating time at 200°C, revealing an increase in resistivity due to oxygen loss. Similar changes in optical absorption (b) and x-ray diffraction data (c) was also measured, demonstrating the profound impact of reversible oxygen loss/uptake on the LSFO thin films. |
This grant from the Petroleum Research Fund has been instrumental in supporting the solid oxide fuel cell research effort within my group. Two graduate students have been partially supported on this project, although one of the students left with a MS degree, which was a minor setback to the project. However, a time extension has been requested and granted. In the following year, work will focus on optimizing and better understanding synthesis of A2BO4 films on YSZ and characterizing their interfacial stability at high temperatures. Additionally, we will further explore the kinetics of the oxidation/reduction of LSFO films, for instance using in situ x-ray diffraction measurements of lattice expansion at elevated temperatures.
Copyright © 2014 American Chemical Society