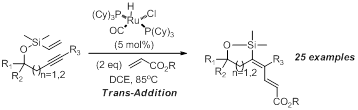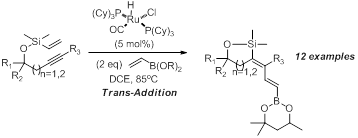58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI150787-DNI1: Transformations Utilizing Ruthenium Hydrides for the Synthesis of Conjugated Systems
Daniel A. Clark, PhD, Syracuse University
The goal of this project was to develop a method for the synthesis of complex dienes. This work has the following objectives: (1) develop a method to incorporate acrylates into complex diene systems, (2) use ethylene to create highly substituted vinyl systems, and (3) explore the use of vinyl boronates to create dienes that contain both a vinyl silane and vinyl boronate moiety.
Findings: Tethered Vinylsilanes and Acrylates
Initially we proposed the use of alkynes that possessed a tethered vinylsilane could be coupled to olefins in the presence of ruthenium hydrides. Realization of our proposed goal provided access to highly substituted dienes that are highly regio- and stereoselective. This reaction proceeds by a rare trans-addition across the alkyne. Our reaction is catalyzed by known ruthenium complex: RuHCl(CO)(PCy3)2. This complex can easily be obtained from ruthenium chloride and is a moderately air and moisture stable bright-yellow crystalline solid. The reaction scheme below offers a general overview of the reaction describe. Both five and six-membered silacycles can be formed in good yield (50-92%). Hindered and non-hindered acrylates participate in this reaction. Further functionalization of the corresponding oxasilacycles was carried out. For example, protio-desilylation, iodination, and subsequent palladium catalyzed cross-coupling events were reported.
Findings: Incorporation of Ethylene
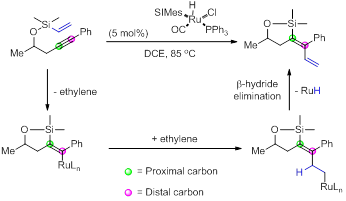
Our studies have expanded to include the use of ethylene as an olefin in the above reaction sequence. Using RuHCl(CO)(SIMes)PPh3 as a catalyst (instead of RuHCl(CO)(PCy3)2) vinyl substituted dienes can be formed without the addition of ethylene. The vinyl group is transferred from the silicon across the alkyne to give the product as described below. Loss of ethylene and formation of a vinyl-ruthenium moiety is followed by an ethylene capturing event that leads to the vinylated diene product. We found that this reaction was highly effective when methyl vinyl ketone (MVK) was used as a co-catalyst. This protocol allows for straightforward entry into these vinylated dienes.
The only observed by-product in this reaction (when conducted in the absence of acrylate) was the six-membered ring product derived through cycloisomerization. Currently we are investigating a more general method for the synthesis of the corresponding cycloisomerization products as they have not been reported previously.
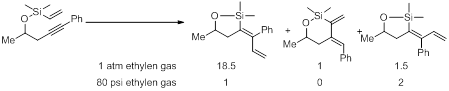
Of particular importance, we also observed the six-membered ring cycloisomerization product formation could be limited by the introduction of ethylene gas (1 atm). However, these conditions gave rise to isomeric products. In all cases the trans-addition was highly favored with cis-addition being the minor product. The cycloisomerization species was present only in trace amounts by nuclear magnetic resonance (NMR) on all cases examined.
We are currently pursuing the effect high concentration of ethylene might have on this reaction. Using 80 psi of ethylene gas under otherwise identical conditions, the cis-addition was favored over the trans-addition product and the cycloisomerized product was not observed by proton NMR. The product ratio has been found to be dependent on electronics; we have preliminary data which suggests and isomerization occurs and this process may be attenuated by electronic effects. We are currently pursuing the exact nature of this isomerization and the mechanistic ramifications thereof.
We are particularly interested in the use of alkynes which have non-aryl functional groups at the alkyne terminus. For example, alkyl groups are of interest as they would grant access to interesting molecular structures not currently available using current methods. In addition, our acrylate studies could not accommodate terminal alkynes. This is one area we hope to improve on using ethylene as an olefin.
Findings: Vinylboronates Containing Dienes
In an effort to broaden the scope of the dienes produced using the above methods we wanted to incorporate vinyl boronates into these systems. Our studies indicated that the hexylene glycol derived vinyl boronate performed well in this transformation. We observe only trans-addition across the alkyne which was anticipated from our prior work with acrylates.
Dienes synthesized with this methodology have been subjected to further functionalization using palladium catalyzed coupling methodology to create higher substituted dienes and trienes. In addition, we have moved beyond simple metal catalyzed methodology and are investigating other methods to selectively functionalize the vinyl silicon in the presence of the vinyl boronate. The realization of this goal would grant access to materials that are bifunctional in nature and offer numerous possibilities for applications.
The funding provided by the PRF has allowed my group to get important preliminary data for submission to other funding agencies. In addition, we made enough progress and obtained results for a manuscript based on our acrylate coupling methodology.
Students conducting research in this area have had the opportunity to get first-hand knowledge of reaction design, optimization and implementation through catalysis. This funding was very important to help the young students (both graduate and undergraduate) in my lab conduct high quality research which we might not have been able to pursue otherwise.
Copyright © 2014 American Chemical Society