58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI1050927-UNI10: Characterizing Synthesis and Ion Transport in Microporous Mixed-Polyhedral Frameworks
Aaron Celestian, PhD, Western Kentucky University
Introduction
Microporous materials have a long and important history in petroleum science. The ion diffusion properties of natural zeolites and their synthetic analogues have been used successfully for catalysis and molecular separation during the petroleum refinement processes. The goal of this research is to understand processes that direct ion diffusion and allow for specific selectivity in heterosilicate microporous titanium/zirconium/niobium silicates, a class of zeolitic analogues. The tools utilized and insights gained into ion diffusion processes will be broadly applicable to other microporous materials and will directly benefit energy and petroleum sciences.
The outcomes of this work show that ion exchange is a multi-step process that results in local conformational changes that not easily modeled using diffraction techniques. This work also shows that effective pore volume can be increased using rare earth elements (REE) in post process of material synthesis. In the case summaries below, REE increase the effective pore volume by reducing the total number of cations in the channel and also moving the charge balancing cation position away from the medial channel position. This may allow enhanced gas sorption and molecular guest interaction with the photo-sensitive REE cations.
Case Summary: Sitinakite
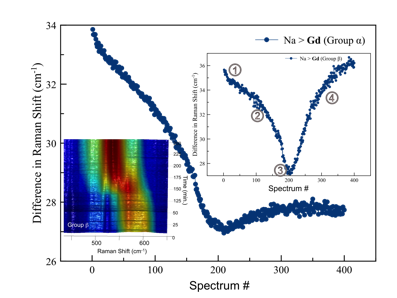
Example plots of REE exchange sitinakite-Na demonstrate that the structural channels become more elliptical as REE cations diffuse into the structure (Figure 1). These channel distortions were marked by changes in the Si-O-Ti and Ti-O-Ti bending modes in the Raman spectrum as peaks move closer relative to each other. The difference Raman shifts plots demonstrate that the REEs induce similar channel distortions, however, the rates and magnitudes of the distortions differ. Distortions of the TiO6 polyhedra in the sitinakite-Na experiments were apparent in higher wavenumber peaks in the Raman spectra. These peaks represent internal stretching modes of the TiO6 and clearly shift as REE cations migrate into the structure. In one case, there may be up to six discrete TiO6 polyhedral changes, and each step has its own kinetic rate law potential and unique chemical functionality. Bonding geometries are currently being modeled via quantum mechanical calculations using VASP.
A band-gap transition occurs at the arrow in Figure 1 as a consequence of molecular stresses in the channel and/or polyhedron. A band-gap transition is also supported by UV-Vis data (not shown), with a band gap shift to higher energy from 4 eV to 4.2 eV. It is unlikely that the band-gap transition is due to a structural symmetry change because time-resolved XRD indicate a constant space group and minimal unit cell volume changes. A more likely explanation is polyhedral distortions/stresses as the exchanged REE electron orbitals interact with framework cations. This would necessitate that the REE reside close to the framework.
Case Summary: Zorite
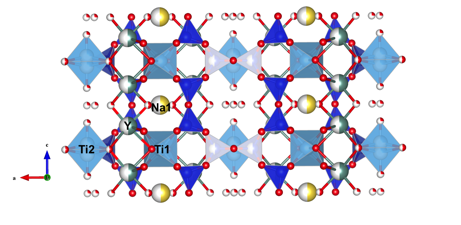
The REE exchange-induced structure transformation in zorite has been successfully modeled. Using a combination of time-resolved Raman spectroscopy, bond-valence theory, and geometry optimization, the exchange mechanisms follow at least two major steps. First, the most dynamic changes in the Raman spectra during REE ion exchange occur around the TiO5 group, approximately located near the Na2 site (Figure 2). The data showed the 773 cm-1 peak (attributed to apical Ti-O stretch) shifted to higher wavenumbers (782 cm-1). This shift is likely due to a shortening of the Ti-O bond as is necessitated if Y bonding occurs at the basal O2– of the TiO5 group. These TiO5 basal O2– show significant distortion during ion exchange as the O-Ti-O bends and Ti-O stretches show a large decrease in wavenumbers (512 cm-1 to 500 cm-1), indicating a lengthening and bending of the Ti-O bond in the TiO5 basal plane. Bond valence sums around the Na1 and Na2 sites support this, as the Na2 site has a lower bond valance sum (BVS of Na2=0.7, Na1=1.0). The new Y site does not directly overlap with the Na2 site, instead it moves to a position higher above the 7-MR, which in-turn forces the Y site closer to the TiO6 group and the Na1 site. The BVS for Y is too high (>4) if it were place in the Na1 site, thus Y cannot directly substitute into the Na1 site. However, the Na from site Na1 may evolve out the structure during ion exchange to maintain charge balance as zorite incorporates higher valance Y3+ cation. This second step during the ion exchange process was to remove Na from site Na1. This step was comparatively slow, and is supported by the reduced rate of change in Raman peak migration, and subtle peak shift of the TiO6 octahedral groups.
H Ion Exchange Summary
Surprisingly, no REE exchange occurred in the H-exchanged, activated zorite, sitinakite, and umbite (zorite-H, sitinakite-H, and umbite-H). Activated microporous materials are typically fast ion conductors for both monovalent and divalent cations. The small H cation should easily diffuse out of the crystal structure in favor of a larger, higher valance cation. However, the REEs are completely rejected by the H-forms of the titanium silicates and zirconium silicates. Our working hypothesis is that it may be a function the overall hydrogen-bond network in the channels and void spaces within the framework. In the as-synthesized forms, Na and K ions are shielded by hydration spheres while they are in the channels, and therefore any exchanging cation would not directly interact with those host cations, allowing ion exchange to proceed. In the activated forms, H is bound to the framework, and is not strongly hydrated.
Figure 1: Results of the difference peak fitting method of time-resolved data for the group α and group β for the Gd exchange into sitiankite-Na. Inset at top right shows step-wise changes during ion exchange as indicated by circled numbers: 1) initial hydration 2) TiO6 polyhedra distort 3) band gap transition 4) TiO6 polyhedra distortion recovers. Inset at bottom left shows birds-eye view of Raman spectra between 450 cm-1 and 650 cm-1.
Figure 2: Proposed structure of zorite-Y. Oxygen is red, titanium is light blue, silicon is dark blue, sodium is yellow, and yttrium is green.
Copyright © 2014 American Chemical Society