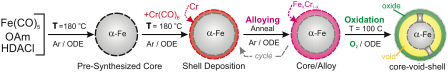
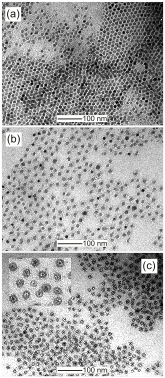
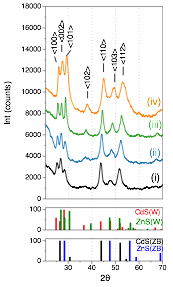
58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051303-DNI10: Investigation of Hydrothermally Processed Nanomaterials for Integration with Third Generation Photovoltaics
Mathew M. Maye, PhD, Syracuse University
Copyright © 2014 American Chemical Society