58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND550829-ND5: Single Molecule Spectroscopy for Characterization of Mesoporous Acid Catalysts
Keith Hohn, PhD, Kansas State University
Dnaiel Higgins, PhD, Kansas State University
The goal of this project was to apply single molecule spectroscopy to study acid sites in heterogeneous catalysts. In the first year, we demonstrated the use of the pH-sensitive dye C-SNARF-1 to probe the acidity properties of microenvironments in mesoporous aluminosilica films. The data revealed significant acid site heterogeneity. We continued this line of research in the second year with a new fluorescent molecule, Violamine R. We have also begun to probe the acidity inside the pores of zeolite crystals.
Single Molecule Studies of Acidity in Aluminosilica Films.
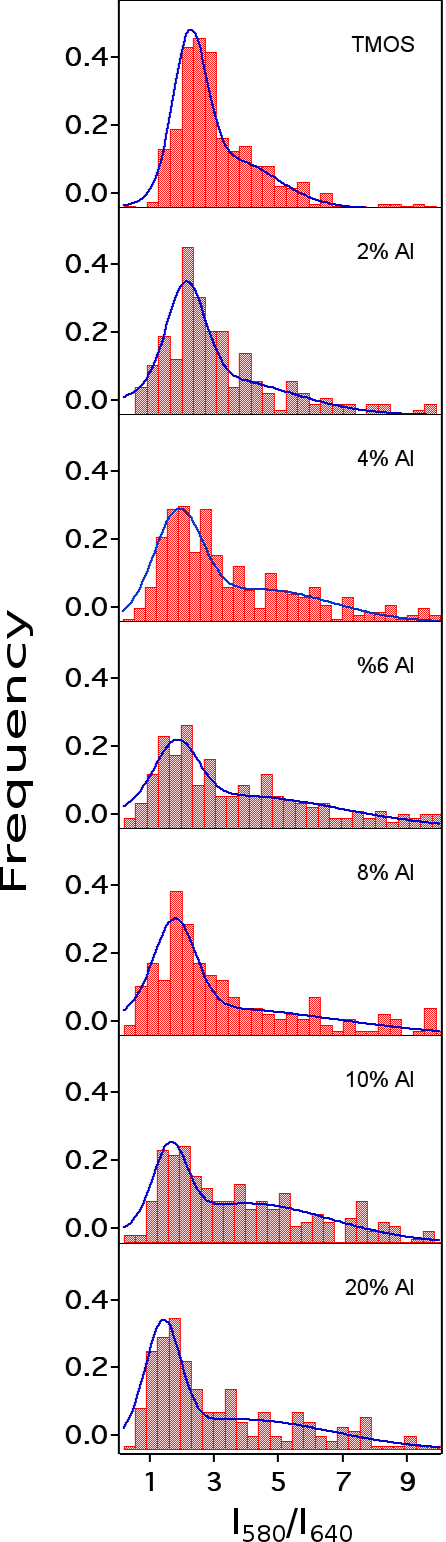
C-SNARF-1 was used to probe the acidity properties of aluminosilica films incorporating 0 to 20 mole percent Al. Acidity was quantified by recording C-SNARF-1 single molecule emission simultaneously in two spectral bands: 580±20nm and 640±20nm. Histograms of the emission ratios were obtained for each film (Figure 1). These data reveal non-Gaussian distributions and are consistent with the presence of at least two distinct environments in some samples.
C-SNARF-1 data obtained from undoped silica was interpreted to reflect the presence of relatively basic microenvironments, having pH ~ 6. Introduction of tetrahedral Al atoms is expected to produce more acidic sites, and this expectation was reflected in the single molecule data: a new peak at higher emission ratios was observed for Al-doped films. The position of the new peak in the Al-doped films (R = 3.0 to 4.4) was concluded to be consistent with a decrease in microenvironment pH to near 4.5, indicating the Bronsted acidity of the films increased with Al content. Unfortunately, C-SNARF-1 was also found to exhibit a non-ideal pH-dependent response, necessitating the use of alternative dyes to better understand the pH of the individual microenvironments.
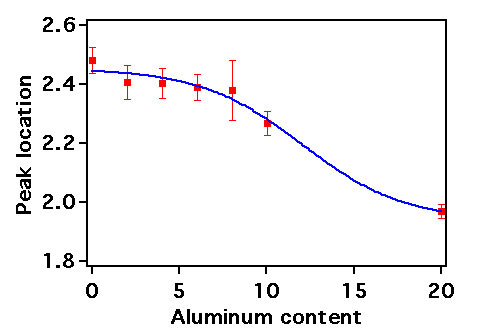
In order to overcome difficulties with C-SNARF-1, Violamine R (VR) was used as an alternative probe of microenvironment pH in mesoporous aluminosilica films. VR is also a dual emission probe, producing emission peaks at 585 nm and 650 nm in basic and acidic environments, respectively. Mesoporous aluminosilica films (0% - 20% Al content) were doped with VR at nanomolar levels. Histograms depicting the Rv values from a large number of single molecules are shown in Figure 2. These data were fit to Gaussian functions and the peak positions are plotted as a function of aluminum content in Figure 3. The VR results confirm the interpretations of the C-SNARF-1 results: the pH of the microenvironments within the films shifts to smaller values as the aluminum content increases. Unfortunately, the results also demonstrate that it is difficult to quantify the local pH by these methods alone.
Nevertheless, the Rv histogram widths do provide additional evidence of microenvironmental properties. Specifically, it is found that the 0% Al and 20% Al samples yield narrow histograms, while the intermediate samples yield broader histograms. VR should yield the broadest histograms for pH 4-6 environments, where the dye is most sensitive. It is concluded that the VR data is consistent with the C-SNARF-1 results: the pure silica film environments have pH ~ 6 (or higher), while high Al content films produce microenvironments with pH ~ 4 (or lower). It is also possible the variation in widths reflects microenvironmental heterogeneity in these materials, with intermediate doping levels producing the most inhomogeneous materials.
The histograms for C-SNARF-1 and VR were different as they have different emission behaviors. C-SNARF-1 has two sensitive regions and better reveals aluminosilica film heterogeneity, while VR exhibits a more monotonic trend in response to pH. Taken together, the results of both dyes provide a better view of microenvironmental acidity and acid site heterogeneity in aluminosilica materials. Both indicate an increase in acidity with greater aluminum doping.
Preliminary Studies of Acidity in Zeolite Pores
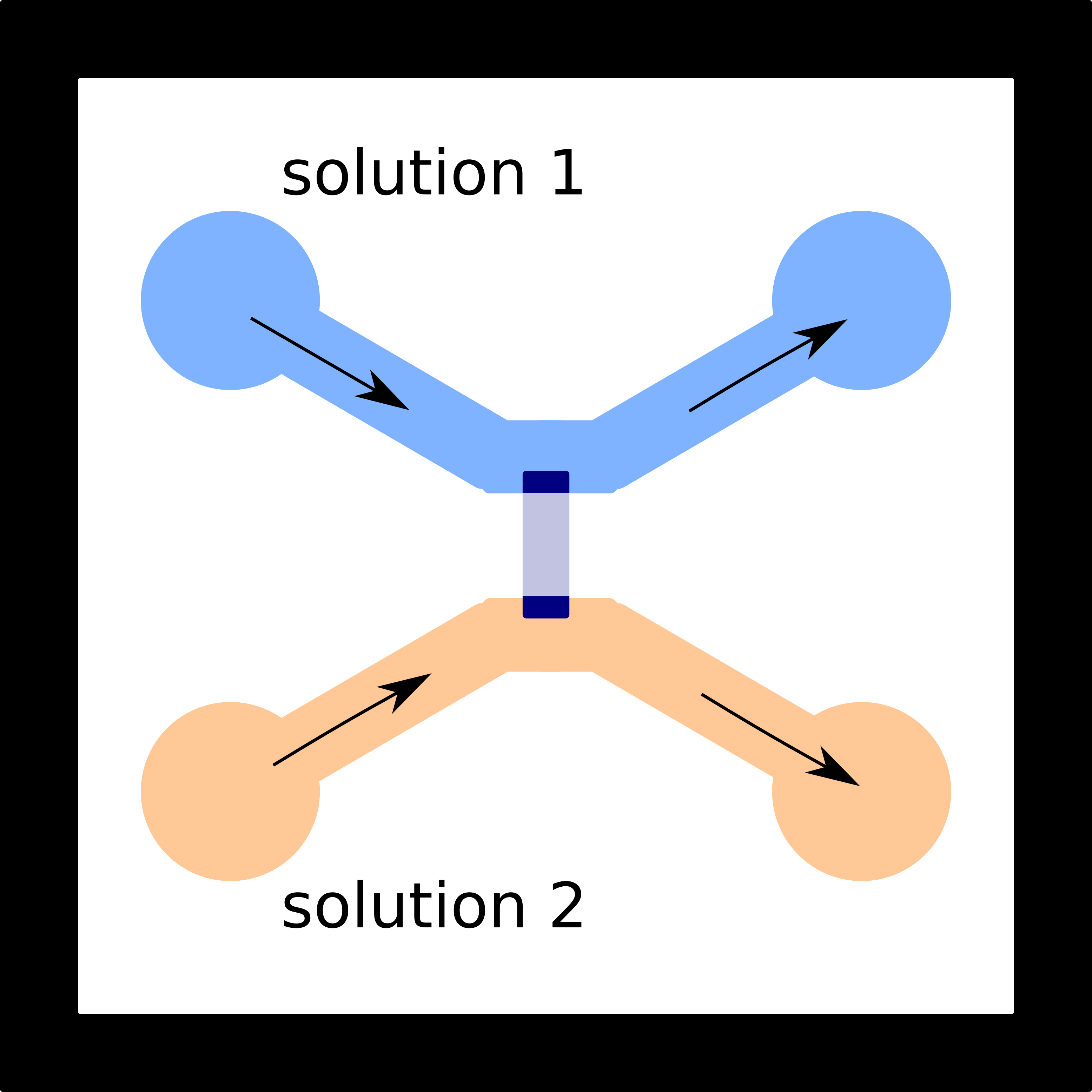
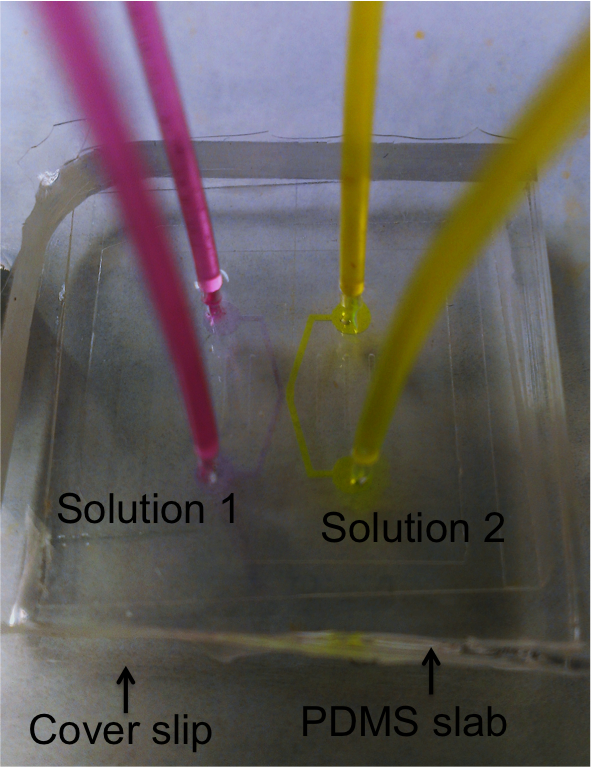
Single zeolite crystals of AlPO4-5 and SAPOs were also investigated. They have the AFI type structure which gives large particle size, one-dimensional pores and large pore openings capable of including fluorescent molecules. The goal was to characterize the interior surfaces of zeolite pores by applying a microfluidic device created by photolithography (Figure 4). Most of the exterior surfaces are covered with cured photoresist mixture (60% ZipconeTM UA/UE and 40% NOATM 74) so only the two ends of the zeolite fiber contact the dye solution. Figure 5 is a demonstration of the device where the two colors demonstrate how two different dye molecules or two solutions of different pH could be flowed into the zeolite. Dye introduced into one of the streams would diffuse into the pores, allowing the local acidity within the pores to be probed.
Unsolved challenges include: 1) the zeolite fibers crack during heating and 2) the similar refractive indices of the zeolite fiber and the photoresist make it difficult to construct a perfect microfluidic structure over the zeolite fiber. Using a gentle heating process and better labeling on the cover slip may resolve these problems.
Impact of Project on Personnel
This project has enabled one of the PIs (Hohn) to expand his research into a new area: use of single molecule spectroscopy to study catalysts. He plans to continue in this research direction, recently submitting a research proposal to the National Science Foundation. This project has also established collaboration between the Hohn and Higgins laboratories that will continue to benefit both researchers. The PhD student (Stella Sun) working on this project received interdisciplinary training in how to conduct cutting edge research. As a result, she has been hired by Intel as a production engineer.
Figure 1. Histograms of C-SNARF-1 emission ratios in mesoporous aluminosilica films.
Figure 2. Histograms of Violamine R emission ratio, Rv, from single molecules in aluminosilica films.
Figure 3. Peak locations of aluminosilica thin films as a function of Al content.
Figure 4. Diagram of the microfluidic device developed for single molecule acidity studies in zeolites. The zeolite crystal is covered with cured photoresist so that the two ends of the crystal are open to the microfluidic channels used for exposure to solution.
Figure 5. Image of a microfluidic device. The solutions were doped with high concentration of dye molecules in order to visualize the flow pattern inside the channels.
Copyright © 2014 American Chemical Society