58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051860-DNI10: Robust Redox Shuttles for Overcharge Protection of Lithium Ion Batteries
Susan A. Odom, PhD, University of Kentucky
Lithium-ion batteries are ubiquitous in consumer electronic devices, yet safety issues and limited lifetimes have hindered their application in large-scale applications. Numerous chemical reactions lead to decreased capacities, as well as hazardous failure mechanisms such as fires and explosions. One of the chemical means that results in limited lifetimes is overcharge, a condition during which the voltage of a battery is raised past the end-of-charge potential of the cathode. Undesirably high voltages lead to overdelithiation of the cathode, decreasing cell capacitance. Additionally, high voltages increase rates of decomposition reactions at the electrode/electrolyte interface, which are often accompanied by temperature increases and pressure build-up, potentially leading to thermal runaway conditions.
Overcharge can be combated using redox shuttles, which act as an internal shunt to prevent electrolyte oxidation by oxidizing themselves. Instability of the oxidized form, usually a radical cation, limits redox shuttle lifetimes and thus the number of overcharge cycles that can be maintained before failure to stabilize (limit) cell voltage. This research project focuses on experiments to help us determine the rules governing the stability of the radical cation forms of redox shuttles in battery electrolytes with the ultimate goal of designing more robust redox shuttle additives for long-term overcharge protection in lithium-ion batteries.
Overcharge experiments limit the pace of progress in identifying promising redox shuttles in that overcharge testing can take several months before completion, so there is a delay in identifying the next generation of targets. Additionally, reversibility by electrochemical techniques such as cyclic voltammetry do not provide results that correlate with redox shuttle longevity. Our initial focus, therefore, in screening redox shuttle candidates, was to identify a new technique to filter unpromising candidates, therefore fabricating and testing fewer coin cell batteries. We analyzed the stability of this oxidized form of the shuttle to determine if a correlation between radical cation stability and number of overcharge exists. We used UV-vis and EPR spectroscopy, two common techniques for analyzing radical cations, to monitor the percent of radical present over time. We studied two series of related compounds: 1,4-dialkoxybenzene derivatives and related heterocyclic aromatic derivatives (Figure 1).
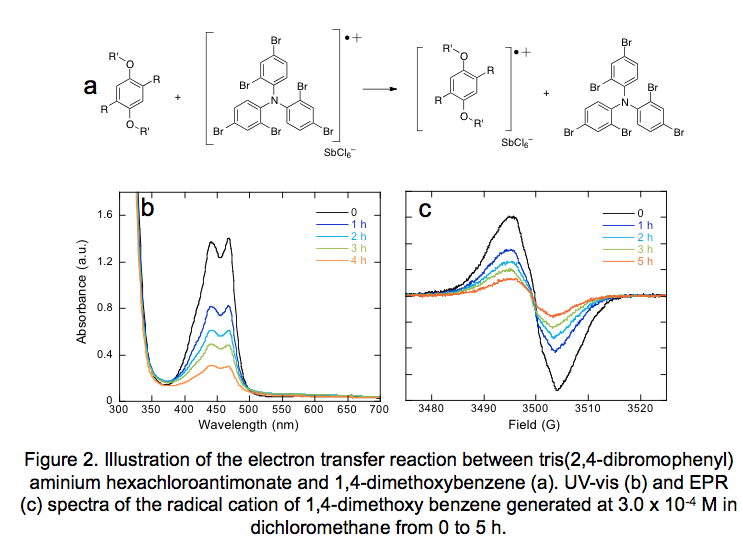
We generated radical cations at 3.0 x 10-4 M in dichloromethane by reacting solutions of redox shuttles with the chemical oxidant tris(2,4-dibromophenyl)aminium hexachloroantimonate, an organic soluble oxidant that can be prepared in two steps from triphenylamine. The dark green color of the oxidant immediately disappears upon electron transfer, creating the neutral form of the oxidant and the radical cation of the redox shuttle (Figure 2a). UV-vis and EPR spectra were monitored over at least 5 h in all cases. UV-vis spectra and EPR spectra of the radical cation 1,4-dimethoxybenzene are shown in Figures 2b and 2c, respectively.
UV-vis and EPR studies were performed on the radical cations of all redox shuttles shown in Figure 1. In most cases, the UV-vis and EPR spectra showed similar trends in decay, comparing the percent of the initial absorbance and area under the integrated spectra, respectively. In Figure 3a-b, plots of the normalized intensity of the original absorbance in UV-vis are shown for both series, showing that the rates of decay vary significantly within 5 h of testing. In Figure 3c-d, a plot of the absorbance intensity at 1, 2, 3, and 5 h vs. number of overcharge cycles is shown. In addition to dichloromethane, a relatively inert solvent for stabilizing radical cations, similar trends in stability were observed for a combination of ethylene carbonate and ethyl methylcarbonate, as well as 1.2 M LiPF6 in ethylene carbonate / ethyl methyl carbonate (not shown), although in cases of some low oxidation potential derivatives, the presence of LiPF6 resulted in radical cation formation whether the oxidant was added or not.
Within the 1,4-dialkoxybenzene series, the redox shuttles show a correlation in radical cation stability and number of cycles of overcharge performance except in one instance. The same is true for the heteroaromatic compounds except for AcPT. With AcPT, we hypothesize that AcPT/AcPTá+ itself is not responsible for overcharge protection, but instead is protected by a decomposition product of AcPT. The change in shape of the UV-vis spectra, rapid decay of AcPTá+ by EPR, change in voltage of overcharge protection over time, and analysis of decomposition products are supportive of this conclusion. We found that AcPTá+ decomposes into parent phenothiazine (PT) as well as oligomeric and polymeric derivatives of AcPT, at least one of which is stable as a radical cation in ambient conditions for days.
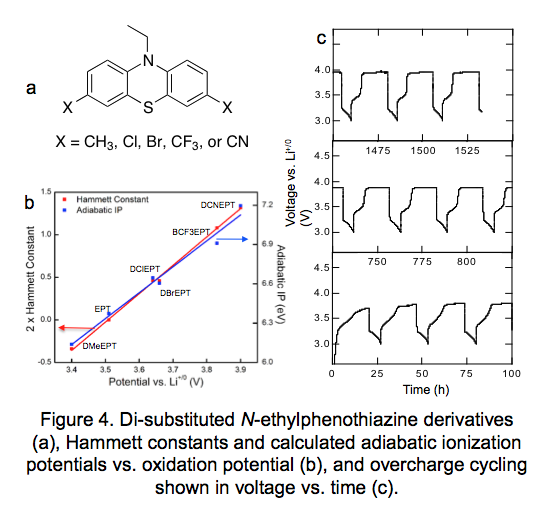
More recently we synthesized di-substituted derivatives of one of the more promising redox shuttles: N-ethylphenothiazine, specifically blocking the position on the aromatic ring para to the nitrogen atoms due to reported dimerization and oligomerization at that position when in the radical cation form. In addition to methyl groups, derivatives with electron-withdrawing groups were also prepared to increase the oxidation potential of the compounds for use with high voltage cathodes (Figure 4a). Trends in oxidation potentials were reliably predicted using Hammett constants and adiabatic ionization potentials from DFT calculations (Figure 4b). While increasing the oxidation potential of a compound often decreases the stability of its radical cation form, we found one derivative with CF3 substituents has remarkable stability in comparison to derivatives with Br or CN groups, which we think is due to the strength of the C-CF3 bond in comparison to C-Br bonds; additionally the derivative with CN groups may be too electron poor with an oxidation potential higher than the CF3 version. The CF3 derivative is currently cycling at cycle number 59 (Figure 4c).
Current work focuses on continued overcharge cycling of new derivatives, optimizing the screening method based on UV-vis spectroscopy, identification of the byproducts of radical cation formation, and design of new targets with specific oxidation potentials, including those based on phenothiazine, phenoxazine, carbazole, and 1,2- and 1,4-dialkoxybenzene derivatives.
Copyright © 2014 American Chemical Society