58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND951641-ND9: Controlling the Partial Coalescence of Particle Stabilized Droplets
Ilona Kretzschmar, Dr., City College of New York, CUNY
David Harbottle, PhD, University of Alberta
Summary
In the previous year of funding, we determined that the transition from partial to complete droplet coalescence for particle-laden (colloidal silica) aqueous droplets with planar hexadecane/water interface depends on particle concentration, size and surface charge (electrolyte concentration). In this funding period, we focused on particle surface wettability (surface functionality) to better understand the effect of particle hydrophobicity on the coalescence mechanism. A titration method was developed that pseudo-quantifies the particle hydrophobicity by the amount of surface hydroxyl groups. In addition, we have begun to study the behavior of plain and surface-modified silica particle stabilized emulsions.
Introduction
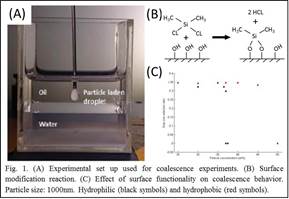
During the latest term of funding, research has focused on the effect of particle wettability (a variable of particular relevance in petroleum processing) on the coalescence mechanism of particle-laden droplets. Preliminary data obtained with the set-up shown in Fig. 1A showed that hydrophobization of the particle surface via silylation (Fig. 1B) leads to the unexpected retardation of partial coalescence (Fig. 1C) to much higher weight fractions (37 vs. 47wt%). The measured difference may result from aggregation of hydrophobic silica when dispersed in a hydrophilic solvent, or from the interaction of particles at the droplet liquid-liquid interface. In order to better understand the observed behavior, a titration method has been developed to quantify the amount of silanol groups on the silica surface, which represents an indirect measure of surface hydrophobicity.
Experimental Details
All experiments described use silica particles (AngstromSphere®, diameters: 250, 500, and 1000nm). Particle batches are treated with 59% nitric acid overnight (unmodified particles). DI water purchased from Fisher Scientific is used as aqueous phase, and hexadecane (analytical grade) without further purification as the immiscible light oil phase. 10-4 M KNO3 is used as base electrolyte in all particle suspensions unless noted otherwise. Particle wettability is varied through a surface silylation procedure with dimethyldichlorosilane in methanol (modified particles).
Titrations without and with SiO2 (1.5 g) particles are performed in the following fashion. 50 ml of DI water is adjusted to pH 4 using 4.2 ml of 12 M HCl. 30 g of NaCl is then added to the solution followed by increasing the total volume to 150 ml by addition of DI water at 25 °C. Subsequently, 0.1 M NaOH is added to the solution in small increments (0.1 ml) until the solution reaches pH 9. The total volume of NaOH added represents the titration volume.
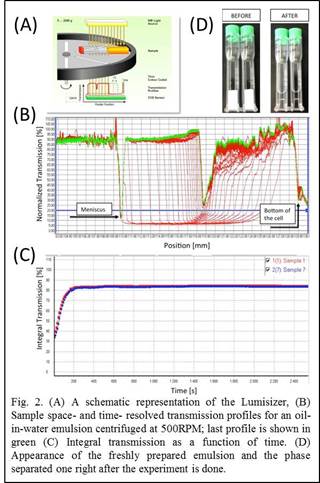
Emulsions of 1:1 water/hexadecane without and with 5w% silica particles (250, 500 and 1000nm) are prepared for stability measurement. Emulsions stability is evaluated by measuring the creaming and sedimentation properties under a centrifugal force, to enhance the destabilization rate. As shown schematically in Figure 2A, the LUMiFuge® rotates samples at a constant angular velocity, while near infrared light is incident on the sample cell. Depending on the turbidity of the sample, a portion of the light is transmitted and detected by a CCD sensor; hence, space- and time-resolved transmission profiles are obtained (Fig. 2B). By integrating the area under the transmission curve, from the bottom of the cell up to the meniscus, one can track the phase separation process in time (Fig. 2C). The increase in integral transmission by time is suggestive of the turbid nature of the freshly made emulsions that become more transparent with phase separation; this phenomenon can be readily observed from the before and after images shown in Fig. 2D.
Results and Discussion
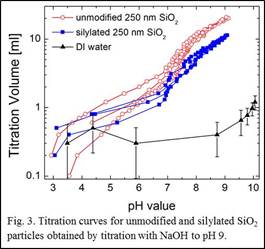
We modified the surface wettability of 250, 500, and 1000nm SiO2 particles using a straight forward silylation method with dimethyldichlorosilane in methanol. Figure 3 shows the results from the titration experiments performed with pure DI water, untreated 250 nm silica, and treated 250 nm silica particles. A clear reduction of the titration volume above pH 7 is observed for the silylated 250 nm particles indicating the reduction of exposed surface hydroxyl groups. Similar trends are observed for the 500 and 1000nm particles.
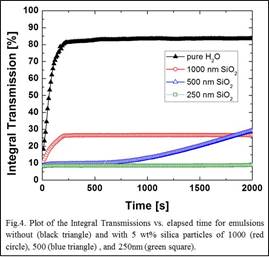
The stability of oil-in-water emulsions stabilized by 5 wt% unmodified silica of particle sizes 250, 500, and 1000 nm is shown in Fig. 4. Addition of particles is shown to substantially increase emulsion stability. More interestingly, the stability is shown to be a function of the particle size with smaller particles preventing phase separation within the experimental condition. This extreme stability can be attributed to the network formation of the particles at the interfaces that causes steric hindrance.
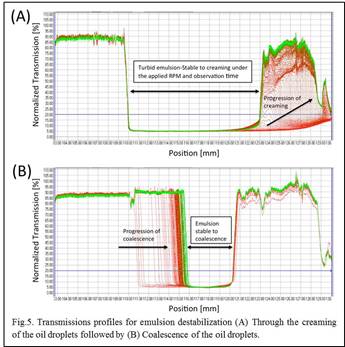
One of the fascinating aspects of the LUMiFuge® is its potential for resolving various instability mechanisms such as creaming, sedimentation, and coalescence by tracking the progression of the transmission profiles as a function of time. In other words, by changing the applied centrifugal force, one might isolate the creaming/sedimentation process from the coalescence event in order to study the two mechanisms separately. In our setup, we have controlled the centrifugation speed (RPM) so as to measure creaming destabilization at lower RPM followed by coalescence destabilization at higher RPM. As shown in Fig. 5A, oil droplets dispersed in the water phase rise to the top of the sample cell (creaming) as evidenced from the increased transmission towards the cell base. With creaming almost complete, as shown by constant cell transmission profiles (green line in Fig. 5A), the RPM is increased to study droplet coalescence (Fig. 5B). Above a critical applied load the particle stabilized networks fracture releasing emulsified oil to form a clear top phase. This technique will be adapted to study the critical loading force necessary to disrupt particle-stabilized, network-stabilized droplets which is of particular relevance in breaking rag and dense-packed layers.
Future Work:
In its second year, the research program has continued to generate interesting and thought provoking results that have contributed to a better understanding of particle-stabilized droplets both at a planar-fluid interface and emulsions.
We will continue our research effort on surface wettability and the distribution of wettability (Janus particles) to understand the critical particle conditions that lead to stable liquid-liquid interfaces. Our knowledge is particularly relevant to petroleum processing where irregular and Janus-like particles are often the cause of emulsion sludge formation.
Copyright © 2014 American Chemical Society