58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI651228-DNI6: Investigating the Role Solute-Solvent Interactions in Asphaltene Nanoaggregation using 2D IR Spectroscopy
Amber T. Krummel, PhD, Colorado State University
Overview
The ACS PRF was used to support one full-time graduate research assistant and their associated running costs. The funding provided to this project has made a significant impact on my student's career, as well as my own. Ms. JenŽe Cyran is now beginning her fourth year of graduate school. Ms. Cyran has been fully supported on this ACS PRF project and thus has benefited from having time in the lab in order to gain traction in her research. She has made significant progress in her research and as a result is working on preparing two manuscripts and attended the 246th American Chemical Society National Meeting in Indianapolis, Indiana this past September. Ms. Cyran presented a poster communicating her work and was able to attend talks relevant to this project during the meeting.
Scientific Progress
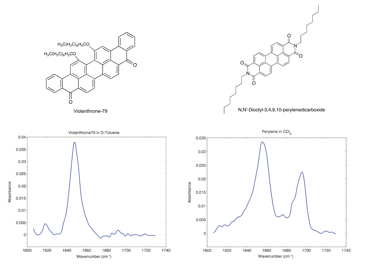
Year 1 of this project was largely focused on building our 2D IR spectrometer and developing the appropriate molecular system for our investigations into asphaltene nanoaggregation. Ms. Cyran worked with a second student in my research group, Jacob Nite, to build our 2D IR spectrometer. In addition, Ms. Cyran identified two compounds that will allow us to answer critical questions regarding asphaltene nanoaggregation. The compounds chosen to model asphaltene behavior are based on previous efforts in the asphaltene literature; violanthrone-79 and N,N'-dioctyl-3,4,9,10-perylenedicarboxide are the two model compounds of interest and their chemical structures and corresponding linear IR absorption spectra are shown in Figure 1.
During year 2 of this project we chose to focus on building our understanding of vibrational coupling in the perylene derivative. These samples are prepared in carbon tetrachloride and contain 10 mM N,N'-dioctyl-3,4,9,10-perylenedicarboxide. All spectra in this report are collected on our 2D IR spectrometer that utilizes Bragg-regime pulse shaping technology; each spectrum in this report is collected with a spectral resolution equal to 8 cm-1. In Figure 2, the linear absorption spectrum and the 2D IR spectrum of N,N'-dioctyl-3,4,9,10-perylenedicarboxide in chloroform is shown. The normal modes in the linear IR absorption spectrum are denoted as A and B. These peaks are likely composed of multiple vibrations that contribute to a Voigt lineshape. The diagonal peaks in the 2D IR spectrum which correspond to the observed peaks in the linear IR spectrum are also denoted A and B. The 2D IR spectrum shown here is indeed noisey, however it exhibits multiple cross peaks that indicate vibrational coupling is present between the observed normal modes. Considering the chemical structure of the perylene derivative, there are four carbonyl bonds and the macrocyclic ring system that are likely to contribute to the observed features in the 2D IR spectrum. A key issue to resolve is how the macrocyclic ring system perturbs the carbonyl stretching vibrational modes. In order to approach this fundamental question, we focused our efforts on a series of quinones containing two carbonyl bonds that are included in a particular ring system. The results of these experiments serve to directly inform the interpretation of the linear IR and 2D IR spectra of the perylene derivative.
In order to extract an accurate local mode description of the carbonyl oscillators in the perylene derivative, the interaction between the carbonyl stretch and the in-plane ring modes must be understood. Benzoquinone, naphthoquinone, and anthraquinone are a series of quinones in which the ring system increases by one six membered, conjugated ring in each case. In each experiment the quinone dissolved in chloroform and the samples were held between two CaF2 plates with a 100 μm thick teflon spacer. Figure 3 contains the chemical structure, linear IR absorption spectrum, and 2D IR spectrum of benzoquinone, naphthoquinone, and anthraquinone from left to right, respectively. The concentration of each quinone was set in order to have a nearly constant optical density for the weakest in-plane ring stretching modes observed between 1560 cm-1 and 1610 cm-1. The concentration of benzoquinone, naphthoquinone, and anthraquinone were 50 mM, 30 mM, and 20 mM, respectively. The diagonal peaks in the 2D IR spectrum for each quinone are labeled according to the corresponding normal modes observed in the linear IR absorption spectrum. Crosspeaks arising from the vibrational coupling between the in-plane ring stretching modes and the modes containing carbonyl stretching motions are labeled as D, I, and M, for benzoquinone, naphthoquinone, and anthraquinone, respectively. By inspection of the data, the strength of the crosspeaks increases as the oscillator strength in the in-plane ring vibrations increases. In addition, the characteristics of the carbonyl stretching vibrations also change dramatically. Currently, Ms. Cyran is working to understand these phenomena from an experimental perspective as well as from a theoretical perspective. We continue to work towards a vibrational coupling model that describes the behavior in this series of quinones, which is the subject of a manuscript that is in preparation.
The overarching goal of this project is focused on developing 2D IR as a tool to probe asphaltene nanoaggregation events. To date, we have built our 2D IR spectrometer based on technological improvements discovered in our group, we have developed a chemical system that will model asphaltene behavior, and we are developing the framework required to accurately describe vibrational interactions between carbonyl stretching modes and in-plane ring modes. Given the many examples of extended ring structures in nature these efforts are certain to impact chemical research beyond the nanoaggregation behavior of asphaltenes. Moreover, the insights gained in this project will inform new directions within my research group.
Figure 1. Chemical structures and linear IR spectra of violanthrone-79 and N,N'-Dioctyl-3,4,9,10-perylenedicarboxide.
|
|
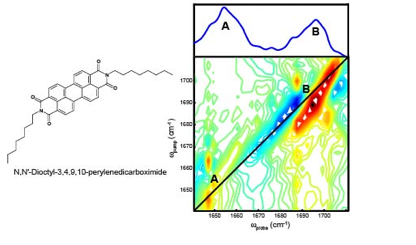
Figure 2. Chemical structure of N,N'-dioctyl-3,4,9,10-perylenedicarboxide (left). The linear IR and 2D IR absorption spectra of N,N'-dioctyl-3,4,9,10-perylenedicarboxide in chloroform (right).
|
|
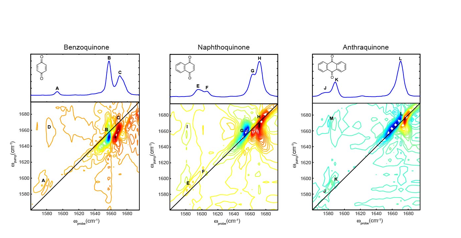
Figure 3. The chemical structures, linear IR absorption spectra, and 2D IR spectra of benzoquinone (left), naphthoquinone (middle), and anthraquinone (right).
Copyright © 2014 American Chemical Society