58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1050859-ND10: Colloidal Synthesis of Zinc Phosphide and Tantalum Oxynitride Nanocrystals for Solar Energy Generation
Preston T. Snee, PhD, University of Illinois (Chicago)
The generation of alternative and renewable energy sources is a significant challenge essential towards sustaining long term economic stability and environmental protection. Solar energy collection and H2 production using water splitting photocatalysts are important processes toward achieving our energy goals. However, most stable oxide photocatalysts absorb ultraviolet as opposed to visible light radiation, while visible light-harvesting alternatives suffer from a variety of problems including instability or suboptimal electronic structure for rapid electron transfer. Many visible light-harvesting systems are also made of highly toxic elements. The results from the past two years of funding from the PRF have addressed several of these issues; in the first year of support we developed novel synthetic techniques to create environmentally–benign tantalum nitride and tantalum oxynitride nanoparticles. In the second year of support methods to create zinc phosphide nanocrystals were developed. The tantalum compounds are thought to be catalytically active for water-splitting, while zinc phosphide may be an important component in next-generation solar cells. The sum total of the accomplishments of the work supported by the ACS PRF can be summarized as follows:
1) In the first year of support, tantalum nitride (Ta3N5) nanocrystals were synthesized using modifications of existing methods to synthesize quantum dots. While we observed the formation of Ta3N5 nanocrystals, ultimately the reaction yields were found to be very low. As Ta3N5 nanocrystals are prone to oxidation, tantalum oxynitride (TaOxNy) was directly synthesized as this material also has catalytic properties. For this study, we developed a sol-gel method that produces large quantities of TaON; furthermore, we demonstrated that the material may be useful for waste-water remediation. Two reports were generated during this time period.
2) In the present period of support, we assisted another PRF awardee, Prof. Delmar Larsen of the University of California at Davis, with two projects examining the photophysics of aluminum phosphide quantum dots. More important to the mission of the PRF, we also studied palladium-tipped CdZnS/ZnS quantum dots have we have found are some of the best materials for photochemically splitting water with light to generate hydrogen gas. We were able to characterize the lifetime of the excited state, and most importantly, the capture of an electron by the metal catalyst. Two publications resulted from this work.
3) Also in the present period of support, zinc phosphide (Zn3P2) nanocrystals have been synthesized using the rapid injection technique. It was found that previous reports of zinc phosphide nanocrystal synthesis were in error and actually were producing large quantities of metallic zinc nanocrystals. It was also found that our Zn3P2 nanocrystals are highly stable against oxidation due to air and water exposure. This research resulted in a manuscript that is presently under review.
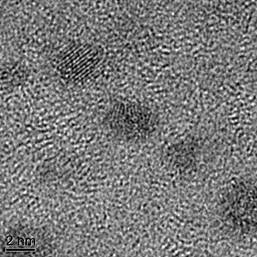
In the 2012-2013 time period, our work was focused mostly on the synthesis of zinc phosphine (Zn3P2) nanocrystals. For this project we used the method of quantum dot synthesis where diethyl zinc (Zn(CH2CH3)2), and tris-trimethylsilyl phosphine, ((CH3)3Si)3P, were injected into a tri-n-octylphosphine solvent at ~300 °C. While zinc phosphide nanocrystals were synthesized, one of the purposes of our proposal was to use less toxic / reactive precursors; as such, we examined the use of zinc stearate in a 1-octadecene solvent. Using this precursor, small (~ 3nm, Fig. 1) nanocrystals were synthesized that were confirmed to be composed entirely of Zn3P2. We also examined the use of alternative phosphorus precursors. Unfortunately, we demonstrated that only ((CH3)3Si)3P could be used to make zinc phosphide, which is unfortunate as tris-trimethylsilyl phosphine is highly toxic and pyrophoric. Regardless, research into alternative phosphorus precursors is ongoing.
Fig. 1. TEM micrograph of a collection of Zn3P2 nanoparticles.
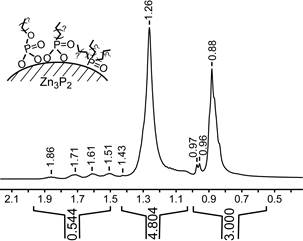
A very interesting aspect of this work concerned our characterization of the surface ligands of the nanocrystals. Conventional wisdom would state that stearic acid coated the nanocrystals. However, NMR investigations revealed that this was not the case (Fig. 2); in fact, trace impurities in the solvents used (most likely phosphonic acids) are the actual surface ligand. Theoretical calculations performed by Prof. Randall Meyer of UIC, also supported previously by the PRF, confirmed that phosphonic acids have very strong interactions with the surface of Zn3P2.
Fig. 2. 1H NMR spectroscopy reveals that Zn3P2 dots are not coated by stearic acid; if true, the resonance at 1.3 ppm would integrate to 32 protons. Inset: proposed Zn3P2 surface ligands.
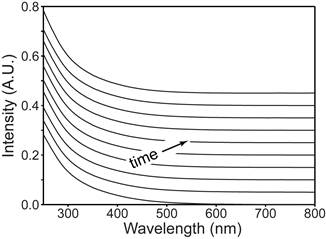
We also found that the material is highly air-stable (Fig. 3), which is very unusual as normally zinc phosphide is prone towards reacting with atmospheric water. This makes the material more desirable for use in solar cell applications. While our manuscript is under review, another report on zinc phosphide synthesis was published where the resulting particles have a thick phosphorus shell, which is highly detrimental for use in photovoltaics. Further characterizations of our nanomaterials reveal that they do not have a phosphorus shell, which indicates that they may be more useful as the active elements in solar cells.
Fig. 3. The absorption spectra of Zn3P2 nanocrystals over a period of three months of constant air and water exposure show no sign of oxidation.
Support from the ACS was instrumental towards developing novel uses for semiconductor nanocrystals regularly synthesized in the labs of the P.I.; several proposals are being prepared based on these works. The preliminary data were taken by a Ph.D. graduate, Dr. Chiun-Teh Ho, whom is now employed by Intel in Hillsborough, OR. Three other students supported by the PRF have graduated; two have Ph.D. degrees and another obtained a Master's Degree. All three are gainfully employed. Two more supported students will graduate within ~2 months with Ph.D. degrees. An undergraduate student, whom is planning to apply to the top chemical engineering graduate schools this semester, prepared a 1st authored manuscript that is presently under review.
Copyright © 2014 American Chemical Society