58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND150808-ND1: New Studies in Alkene 'Pseudohalogen' Difunctionalization
Thomas Lectka, PhD, Johns Hopkins University
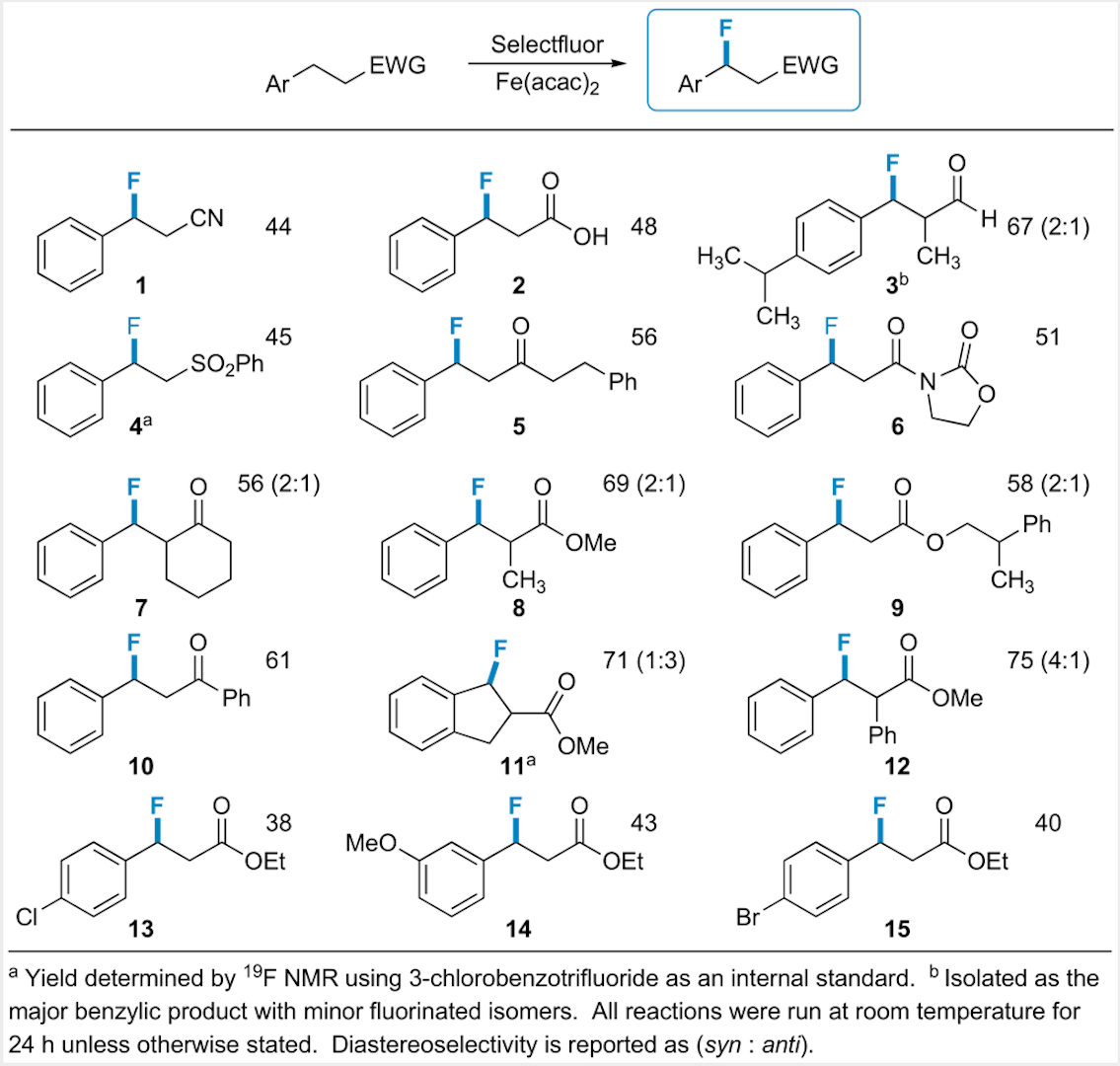
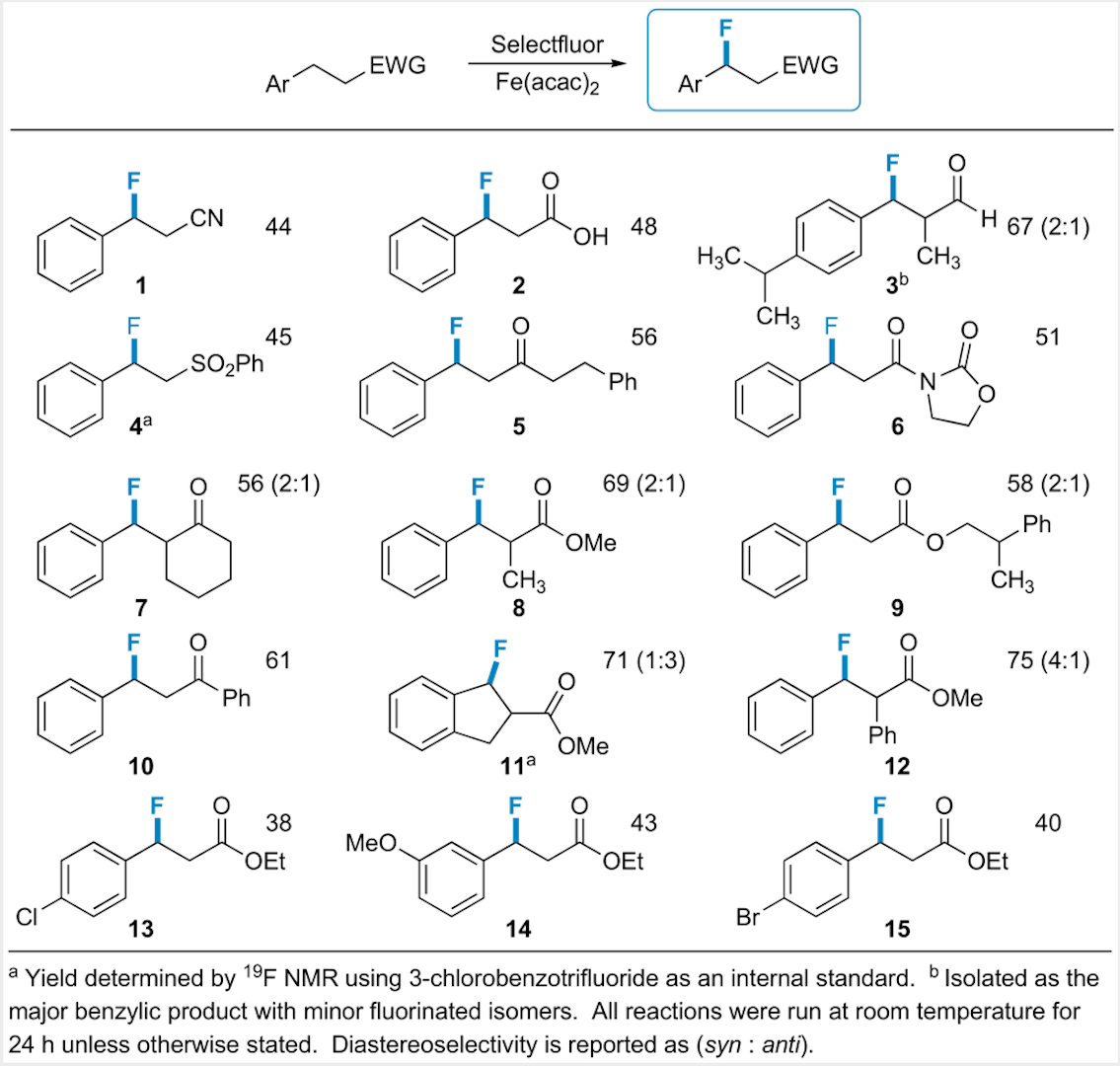
In the past year we havedeveloped the first widely applicable and general catalytic benzylicfluorination method. Our procedureinvolves stoichiometric Selectfluor as the fluorinating agent and Fe(acac)2as the catalyst. This methodshould provide an easy, versatile route to pharmaceutically active fluorinatedcompounds:
More recently we have refined themethod to afford products that are the synthetic equivalent of the conjugateadditon of fluoride, an otherwise difficult process to effect under mildconditions in moderate to good yields:
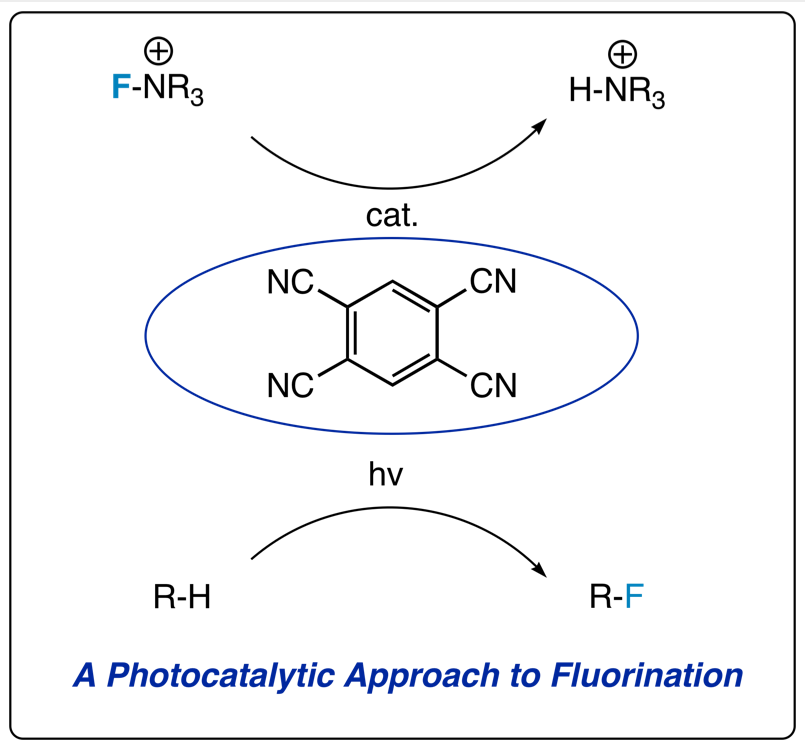
We have also very recentlydiscovered a new procedure for catalytic alkane fluorination involving aphotosensitizer and Selectfluor. The photocatalyst, 1,2,4,5-tetracyanobenzene, effects the formation ofradical cations and putative radicals that can be fluorinated by Selectfluor:
One showcase example of this newtechnology is the phtocatalyzed fluorination of santonin, a natural productthat ordinarily undergoes a photochemical rearrangement:
The next (extension) year shouldbring about another set of exciting new discoveries in this nascent area ofresearch.
Copyright © 2014 American Chemical Society