58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND651898-ND6: Understanding Interfacial Phenomena Related to Enhanced Oil Recovery
Jeffrey R. Errington, PhD, State University of New York at Buffalo
The goal of this project is to develop a better understanding of wetting phenomena in rock-oil-water systems. We are particularly interested in the manner in which various components within crude oil impact wetting behavior. Many have speculated that the heavy asphaltene fractions of crude oil are responsible for altering the wettability of a rock from preferentially “water-wet” to preferentially “oil-wet”. It has been suggested that during the aging process charged components within the asphaltene fraction, such as acids and bases, adsorb onto the rock surface, thereby changing the surface chemistry of the rock. The specific aims of this project include (1) developing a means to compute the wetting properties of immiscible liquids (e.g. oil and water) near a solid surface (e.g. rock) via molecular simulation and (2) apply these techniques to quantify the extent to which carboxylic acids, which are commonly found within the heavy asphaltene fractions of crude oil, alter the wetting properties of the rock-fluid interface. During the first year of the project we have developed appropriate simulation strategies and have demonstrated their efficacy for simple systems. In addition, we have advanced a series of baseline calculations with a realistic model for water at a silica surface.
Wetting of Immiscible Liquids
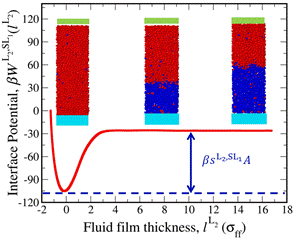
We developed a means to compute the interfacial properties of immiscible liquids at a solid surface via molecular simulation. Our end goal is to use these techniques to determine how the interfacial properties of oil-water mixtures, such as the oil-water interfacial tension and oil-water-rock contact angle, evolve with temperature, pressure, and physical characteristics of the substrate. The approach that we pursued is based upon the so-called interface potential, which provides the surface excess free energy as a function of the thickness of a fluid film in contact with the substrate. For immiscible liquids, we examine either the growth of a liquid phase L2 from the substrate S in a mother liquid phase L1 or the growth of the L1 phase from the substrate in a mother L2 phase. Such curves provide direct measures of the relevant spreading coefficients. Straightforward manipulation of these coefficients provides the interfacial tension and contact angle. In addition to this “direct” method, we also developed schemes to trace the evolution of interfacial properties with temperature or pressure.
We tested the efficacy of these methods with two Lennard-Jones systems. We provide results for a symmetric binary system (components 1 and 2 are identical with a weak energetic cross interaction). The figure below shows the interface potential related to the growth of the L2 phase from the substrate in a mother L1 phase. The difference between the minimum and the plateau reveals the spreading coefficient.
Further calculations were completed to construct the pressure dependence of interfacial properties. The figure below shows the pressure dependence of the liquid-liquid interfacial tension and the contact angle. As one intuitively expects, the interfacial tension increases with increasing pressure. Due to the symmetric nature of the fluid, the cosine of the contact angle should evaluate to zero regardless of pressure, which is what we observe.
In the near future, we will apply these techniques with realistic models for water and n-decane (a model oil) at a silica surface.
Wetting of Water on Silica Surface
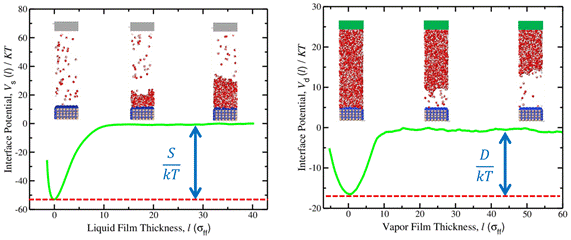
We have initiated a series of baseline calculations to assess the temperature dependence of the wetting properties of water at a silica surface. We began the calculations by constructing interface potentials at a relatively high temperature of 500 K. The figure below provides “spreading” and “drying” interface potentials. The spreading potential provides the surface excess free energy related to the growth of a liquid film from the substrate in a mother vapor background. The drying potential provides the surface excess free energy related to the growth of a vapor film from the substrate in a mother liquid background. Collectively, these coefficients enable us to deduce the liquid-vapor surface tension and contact angle at the conditions studied. We are now completing calculations that will enable us to construct the temperature dependence of these interfacial properties. We will then examine the extent to which carboxylic acids adsorbed to the surface modify the wetting properties.
Training
One Ph.D. student, Wenjing Guo, has been fully supported (stipend and tuition) by this project. Wenjing recently began the third year of our graduate program. This past academic year she has focused almost exclusively on research activities. She has learned how to develop Monte Carlo codes, perform simulations, and analyze results. She completed the work related to the wetting of water on silica noted above.
Copyright © 2014 American Chemical Society