58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1052475-ND10: Structure and Dynamic Studies in Metal-Organic Materials
Tomislav Friscic, PhD, McGill University
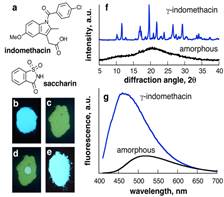
Research direction 1. As our first entry into using fluorescence emission spectroscopy to monitor transformations of solids we addressed the change in solid-state structure of simple fluorescent organic molecules. In order to make initial studies as broadly relevant as possible, we investigated molecules that are also of pharmaceutical relevance. The drug indomethacin has proven to be a suitable model as it provides multiple crystalline forms (polymorphs, cocrystals, solvates) and an amorphous form that is sufficiently stable for handling. We demonstrated that each of these forms can be distinguished by its characteristic fluorescence signature (Figure 1). Moreover, we demonstrated a technique for in situ and real-time monitoring of solid-state fluorescence that utilizes a conventional well plate reader with a thermostated 96-well plate cell. Our first published results1 on using this technique to monitor the temperature- and humidity-dependent recrystallization of amorphous indomethacin revealed sigmoidal kinetics, consistent with the Avrami model for 2-dimensional crystallization. This represents the first application of solid-state fluorescence spectroscopy for real-time monitoring of solid-state transformations. It is notable that the outcomes of our measurements are consistent with previous studies of using Raman spectroscopy.2 This research is being continued through studies of pharmaceutical cocrystallization.
Figure 1. (a) Model molecules indomethacin and saccharin; fluorescence of powdered: (b) crystalline and (c) amorphous indomethacin; (d) partially re-crystallized amorphous indomethacin after adding a drop of CH2Cl2 and (e) indomethacin:saccharin cocrystal; (f) powder X-ray diffraction patterns and (g) fluorescence emission spectra of crystalline and amorphous indomethacin.1
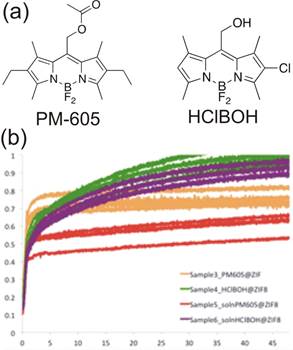
Research direction 2. We also used real-time fluorescence measurements for monitoring MOF formation. The first results of this work are being written up for publication. As fluorescent MOFs are not readily accessible and covalent synthesis of BODIPY-based MOF components is synthetically demanding (in progress, see 3 below) we devised a method to impart fluorescence to MOFs by entrapment of readily available BODIPY dyes within the pores of a MOF as it is being synthesized. We utilized the BODIPY dyes HClBOH and PM605 (Figure 2a) as guests for inclusion in the popular ZIF-8 (commercial Basolite Z1200®).
The largest diameters of the two BODIPY dyes were 9 Å-10 Å, suggesting they are small enough to fit into the 11.6 Å-diameter pores of ZIF-8 but would not be able to leach out through the 3.4 Å pore window. Entrapment of the BODIPY dyes was explored in the solvent-free synthesis of ZIF-8 using the new synthetic methodology of "accelerated aging",3 developed by the Friščić group for solvent-free and low-energy synthesis of metal-organic materials from metal oxide feedstock. Accelerated aging of zinc oxide, 2-methylimidazole and a salt catalyst in the presence of a small quantity (0.5 mol%) of one of the two BODIPY dyes yielded ZIF-8 materials whose structure was confirmed through X-ray powder diffraction. The materials exhibited fluorescence interpreted as a result of dye inclusion. Absorbance measurements indicate that dye inclusion is stable, with 99% of PM-605 and 92% of HClBOH retained in the materials after washing. Monitoring the time-dependent change in fluorescence during accelerated aging revealed a sigmoidal increase within the first 2-5 hours (Fig. 2), interpreted by the inclusion of individual dye molecules into the pores of the nascent framework, which eliminates close contacts and self-quenching encountered in pure solid dyes. Although fluorescence emission reached a maximum within hours, X-ray powder diffraction revealed unreacted starting materials, suggesting that fluorescence spectroscopy provides information only on the course of molecular inclusion, rather than the overall extent of ZIF formation. We believe that fluorescence and X-ray diffraction measurements show that the inclusion of dyes in the growing ZIF takes place only on the surface layers of the MOF, possibly leading to a core-shell structure in which the core is a ZIF and/or a metal oxide and the shell is the ZIF rich with BODIPY guests. This will be verified through fluorescence microscopy and electron microscopy studies of fluorescent ZIF particles.
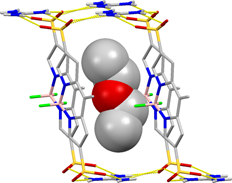
Research direction 3. The synthesis of new BODIPY ligands for MOF synthesis is synthetically demanding. Consequently, we explored the simpler synthesis of BODIPY building blocks for open hydrogen-bonded frameworks. We used the readily prepared BODIPY-disulphonic acid as a building block of guanidinium sulfonate frameworks.4 The first result from this study revealed a three-dimensional hydrogen-bonded framework (Figure 3), with included diethylether guest. Fluorescence and guest sorption properties of this material are being investigated.
Figure 3.Fragment of the hydrogen-bonded open framework found in the guanidinium BODIPY-disulphonate.The methyl groups of the BODIPY dye have been omitted for clarity and the included ether molecules is shown using a space-filling model.
Copyright © 2014 American Chemical Society