58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND550780-ND5: Targeted Delivery of Organometallic Complexes to Low-Coordination Sites on Oxide Surfaces
Lisa McElwee-White, PhD, University of Florida
Jason F. Weaver, PhD, University of Florida
Heterogeneous catalysts will play a central role in meeting global energy needs. Unfortunately, conventional methods for synthesizing oxide-supported metal catalysts suffer from limited control over the structure of the active metal species. Our proposed catalyst synthesis method involves selective adsorption of organometallic complexes onto low-coordination sites of oxide surfaces, followed by removal of the ligands using non-thermal oxygen and hydrogen plasmas at temperatures low enough to preserve the original binding sites of the metal species. The hypothesis is that alcohols and amines will exhibit a preference for binding and reacting at low-coordination step and kink sites of TiO2 and SrTiO3 surfaces and that this can be used to guide the placement of metal atoms on the oxide support.
During Year 2, we first investigated the (12 2 1) SrTiO3 (STO) surface in preparation for studying the adsorption and decomposition of CpRu(CO)2CH3 (1) as a non-functionalized model compound for placement of Ru catalytic sites. the ideal STO(12 2 1) surface is characterized by (100) terraces with a periodic step-kink structure that consists of (111) and (110) facets that are two and one lattice constants wide, respectively (Figure 1a). The student cut and polished STO(12 2 1) samples and annealed a sample extensively in ultrahigh vacuum (UHV) to achieve sufficient conductivity for surface structural characterization using low energy electron diffraction (LEED) and scanning tunneling microscopy (STM). LEED patterns obtained from the annealed sample (Figure 1b) indicate that the STO(12 2 1) samples have the expected crystalline surface structure with a periodic arrangement of kinks and steps.
| |
Figure 1. (a) Side and top view of the STO(12 2 1)-SrO surface. The terraces are (100) oriented, the steps consist of (110) and (111) facets and the kinks involve the intersection of the three low-index facets. (b) LEED pattern (E = 95 eV) obtained after annealing a STO(12 2 1) surface in UHV. The LEED pattern exhibits a square arrangement of spots (yellow outline) due to diffraction from (100) terraces, while diffraction from the periodic kink-step structure gives rise to spot splitting (circled in red) at an angle relative to the (100) lattice vectors. | |
We then investigated the adsorption and decomposition of CpRu(CO)2CH3 (1) on STO(12 2 1) using temperature programmed reaction spectroscopy (TPRS) and X-ray photoelectron spectroscopy (XPS). Complex 1 was adsorbed onto the STO surface using a directed doser in the UHV chamber that attaches to a variable leak valve. Evacuating and heating the sample vial to 45 oC produced sufficient vapor pressure from solid 1 to deliver the complex into the UHV chamber.
Complex 1 adsorbs readily on STO(12 2 1) at about 150 K. TPRS shows that the Ru complex desorbs from a multilayer at about 230 K without reacting, while desorption from a monolayer gives rise to a broader desorption feature between 240 and 330 K. The monolayer desorption feature reaches saturation after the multilayer is well developed, demonstrating that 1 populates the monolayer and multilayer nearly simultaneously. Such behavior is indicative of either weak 1-STO interactions relative to the intermolecular 1-1 interactions, or limited surface mobility of 1 at the temperatures studied. The TPRS results reveal that only a small quantity of the adsorbed 1 decomposes on the STO(12 2 1) surface during heating. We observe only small desorption peaks between 300 and 400 K at m/z fragments at 31, 59 and 72 which are inconsistent with fragmentation of 1 in the mass spectrometer ionizer. The exact identity of the product(s) is not yet known, but a 31 amu fragment is characteristic of a primary alcohol and might form by reaction between 1 and water that adsorbs in small amounts from the background. The low reactivity that we observe is consistent with decomposition of the adsorbed Ru complex at a minority site on the surface, such as kink sites.
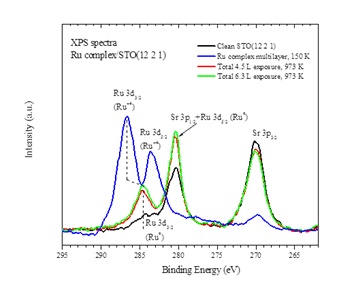
Figure 2: XPS spectra of Ru 3d and Sr 3p peaks obtained from clean STO(12 2 1) and after adsorbing or decomposing 1.
|
We also used XPS to investigate the uptake of Ru onto the STO(12 2 1) surface. In these experiments, we repeatedly prepared multilayers of 1 at 150 K followed by heating to 973 K. The XPS data indeed shows that only small amounts of metallic Ru remain on the surface after a single adsorption-heating experiment, which is consistent with the TPRS results, but that Ru does accumulate after several cycles. In Figure 2, we show the Ru 3d and Sr 3p peaks obtained from the clean STO(12 2 1) surface and after adsorbing or decomposing the Ru complex. A multilayer of 1 significantly attenuates the Sr 3p peak, as expected, and yields Ru 3d peaks with binding energies that are consistent with the formal Ru4+ state, indicating that 1 adsorbs intact on the surface at 150 K. After heating, the Ru 3d peak intensity diminishes significantly and shifts to lower binding energies, thus indicating that a fraction of the Ru complexes decompose to surface Ru during heating. Several adsorption-heating cycles were required to achieve an appreciable increase in the Ru 3d peak intensities. Overall, the TPRS and XPS experiments demonstrate that 1 has a low reactivity on STO(12 2 1), but that repeated adsorption-heating treatments produce measurable quantities of metallic Ru. The low reactivity may indicate that 1 tends to react on surface defects (kinks and steps). To further test this hypothesis, we plan to use STM to image the STO(12 2 1) surface after the adsorption of different Ru complexes, in an effort to determine, via direct imaging, if the complexes tend to bind selectively on surface kink sites.
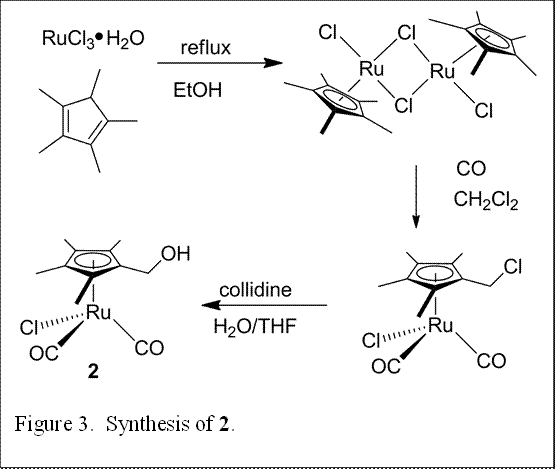
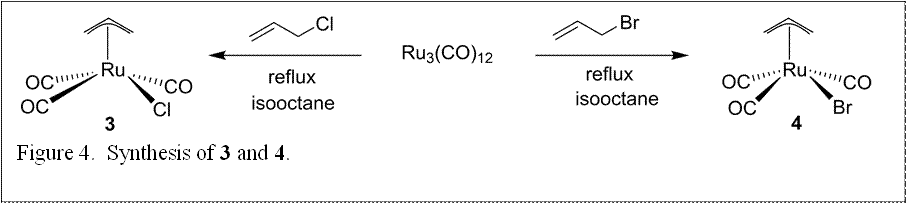
Concomitant with experiments on 1, synthesis of the hydroxyl-substituted derivative 1 (Figure 3) was scaled up for future experiments to probe the effect of a tethered alcohol functionality. Additional synthetic efforts resulted in preparation of the allyl substituted derivatives 3 and 4 (Figure 4), in which the η5-Cp ligand is replaced with the more easily removed η3-allyl. Surface deposition experiments will be continuing with 2-4.
Copyright © 2014 American Chemical Society