58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1052640-DNI10: Stabilized Nanoparticle Partitioning and Interfacial Behavior in Oil-Brine Systems
John Fortner, PhD, Washington University in St. Louis
The overarching project goal is to fundamentally develop and experimentally describe engineered (T1/T2 active) nanoparticle interfacial properties and behaviors in controlled oil-water (brine) systems. Technical project tasks are organized (over a two-year project timeline) into six interrelated foci: 1.) Synthesis and characterization of model libraries of aqueous-stabilized engineered nanoparticles; 2.) Evaluation of particle stabilities (subsets from library) in aqueous/brine systems 3.) Quantification of oil-brine particle partitioning with model and actual crude oil phases; 4.) Enthalpic surface - particle analyses using a quartz crystal microbalance (with dissipation monitoring) with hydrophobic (oil-like) and hydrophilic (sand) surfaces; 5.) Characterization of interfacial (surface) tension and contact angle of oil-water-particle mixtures 6.) NMR evaluation of selected, brine stable particles for their effectiveness as T1/T2 contrast agents.
Year 1 Progress
In accordance with the Project Schedule, Year 1 was heavily focused on Task 1., nanomaterial core development/synthesis, surface coating evaluation/development, and material characterization; Task 2.) Nanoparticle aqueous/brine stabilities; and Task 3.) QCM-D particle – surface studies. While, there was a need for method development in Project Year 1 – especially for nanomaterial synthesis/development, considerable progress was made for scheduled Task and all are on, or ahead, of the overall project schedule.
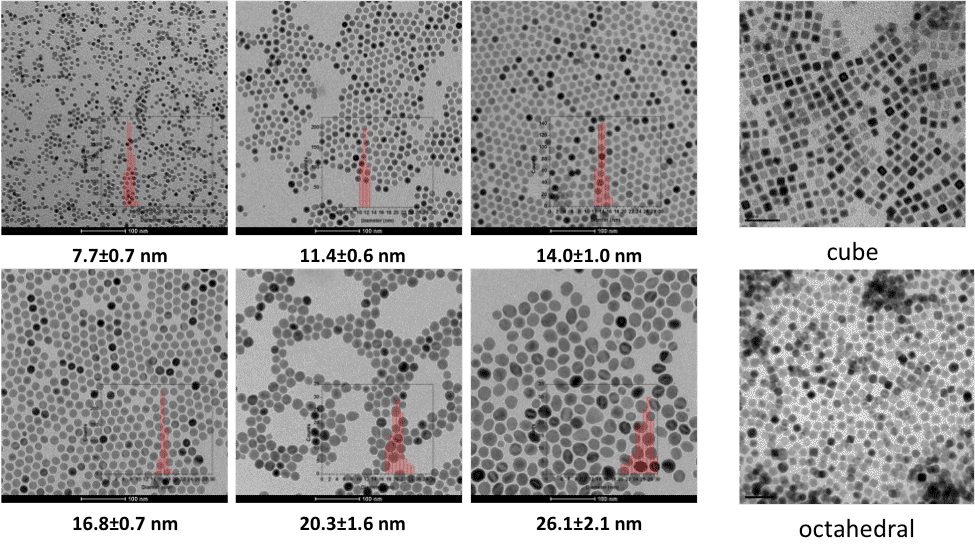
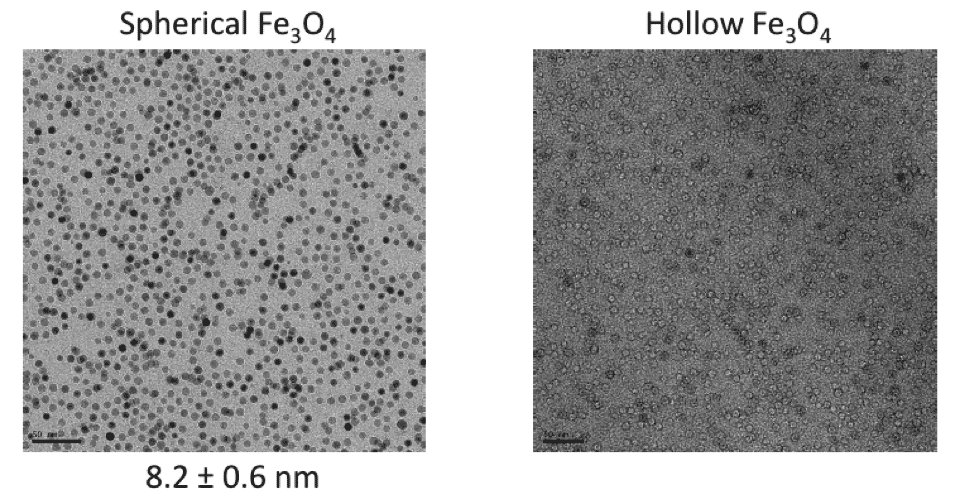
Task 1 Progress Overview. Complete model libraries of water stabilized, engineered nanoparticles were developed with defined size, shape, compositions, and variable surface coatings. Sufficient materials were produced, characterized and organized for all project related future (Year 2) experiments. The material library includes: monodispersed, single domain, Fe3O4 (magnetite) nanocystals (T2 active) with specific sizes of 8-27 nm and shape (spherical, cube and octahedral) (Figure 1.); Single domain, monodispersed manganese oxide (Mn3O4) nanocrystals (T1 active) with specific sizes of 6-25 nm (spherical) (image not shown); 9-10 nm, monodispersed mixed ferrite nanocrystals (T1/T2 active) with varying iron:manganese ratios (9:1 – 1:9 Fe-Ox/Mn-Ox) (Figure 2); 'hollow' monodispersed, shell- metal oxide, structures (newly developed) (Figure 3), which provide a unique and direct avenue to explore enhanced surface energies, differential magnetic response and material density profiles. Methods are available upon request and/or as described in the project proposal.
A number of surface coating strategies, including bi- and single-layers, were explored and optimized for (particle) aqueous transfer. For bilayer passivation strategies, oleic acid acts as the stabilizing base layer (occurring during initial synthesis) and a progression of second layers (as organic acids, surfactants and polymers, which allow for aqueous stability) were evaluated. Positively charged surfactants evaluated include: hexadecyltrimethylammonium(bromide) (CTAB), dodecyltrimethylammonium(bromide), myristyltrimeylammonium(bromide), trimethyloctadecylammonium(bromide). Negatively charged surfactants, polymers and organic acids included: sodium dodecyl sulfate (SDS), (sodium)dodecylbenzenesulfonate, (sodium)hexadecyl sulfate, (sodium)octadecyl sulfate, N-laurylsarcosine, lauric acid, (sodium)stearate, sodium decanoate, sodium myristic acid, sodium palmitate, sodium ricinolate, and sodium monodedecyl phosphate. Zwitterionic surfactants evaluated: N,N-dimethyl-N-docylglycine betaine. Depending on the transfer conditions (time, sonication amplitude, constituent ratios), every strategy/combination tested (above) could be used to produce monodispersed (as evaluated by DLS, TEM), stable nanoparticle suspensions in water (all tested with 8 nm, monodispersed Fe3O4 transferred from hexane to water). Additionally, sterically stable particles (8 nm Fe3O4 and 10 nm Mn3O4) have been achieved using an Igepal series (CA420-720, Sigma Aldrich) and polyethylene glycol (PEG).
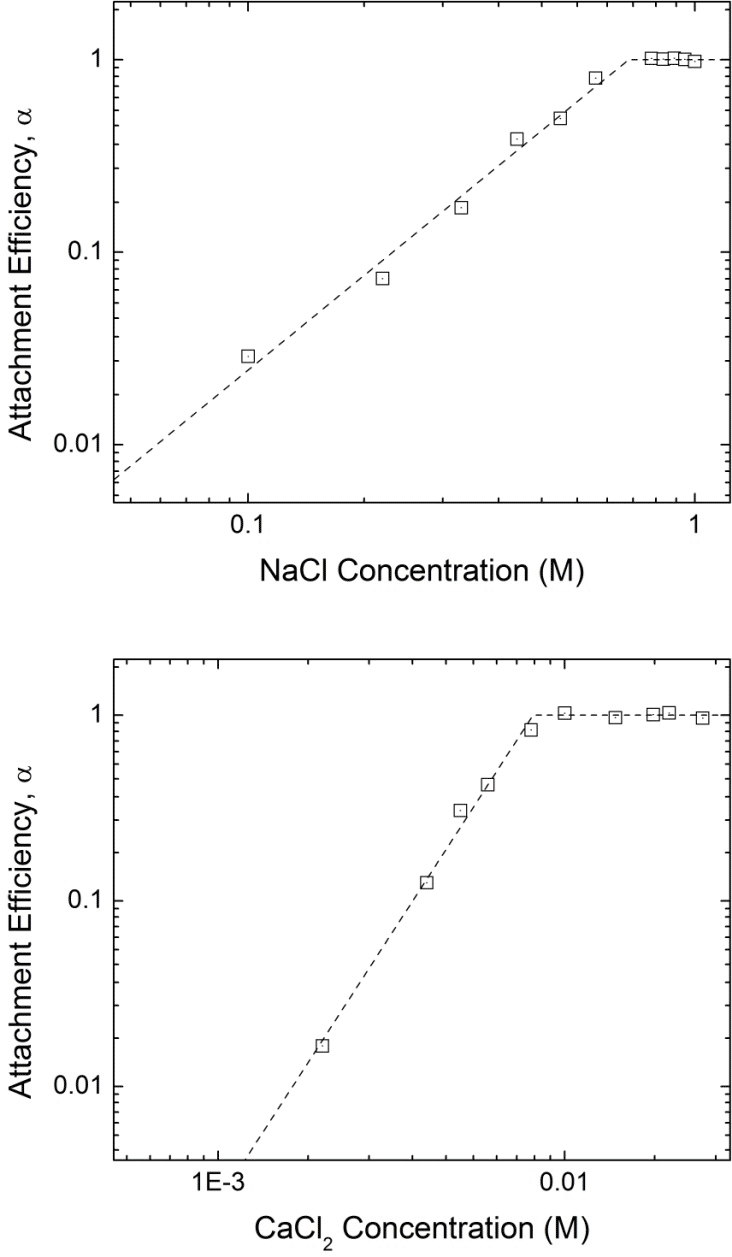
Task 2. Progress. The stability of 8 nm Fe3O4 (oleic acid double layer stabilized in water, 7.5 mg/L) in the presence of varied ionic strength (varied by concentration and type, NaCl and CaCl2) was evaluated via the tendency of particles to aggregate. For NaCl (i.e., < 700 mM), the aggregation rate increases with increasing NaCl concentration eventually reaching a critical coagulation concentration(CCC) 'plateau' of 710 mM. Similarly, the aggregation kinetics of iron oxide nanocrystals in the presence of CaCl2 follows the same trend reaching the CCC at 8.1 mM (Figure 4). The ratio of CCC values calculated (8.1/710) is proportional to z-6.45, where z is the valence of the calcium counterion (i.e., z=2). This is in good agreement with the Schulze-Hardy rule that the CCC ratio for CaCl2 and NaCl should be z-6 for particles with large ζ-potentials. Similar studies are ongoing for selected particle cores and surface coatings.
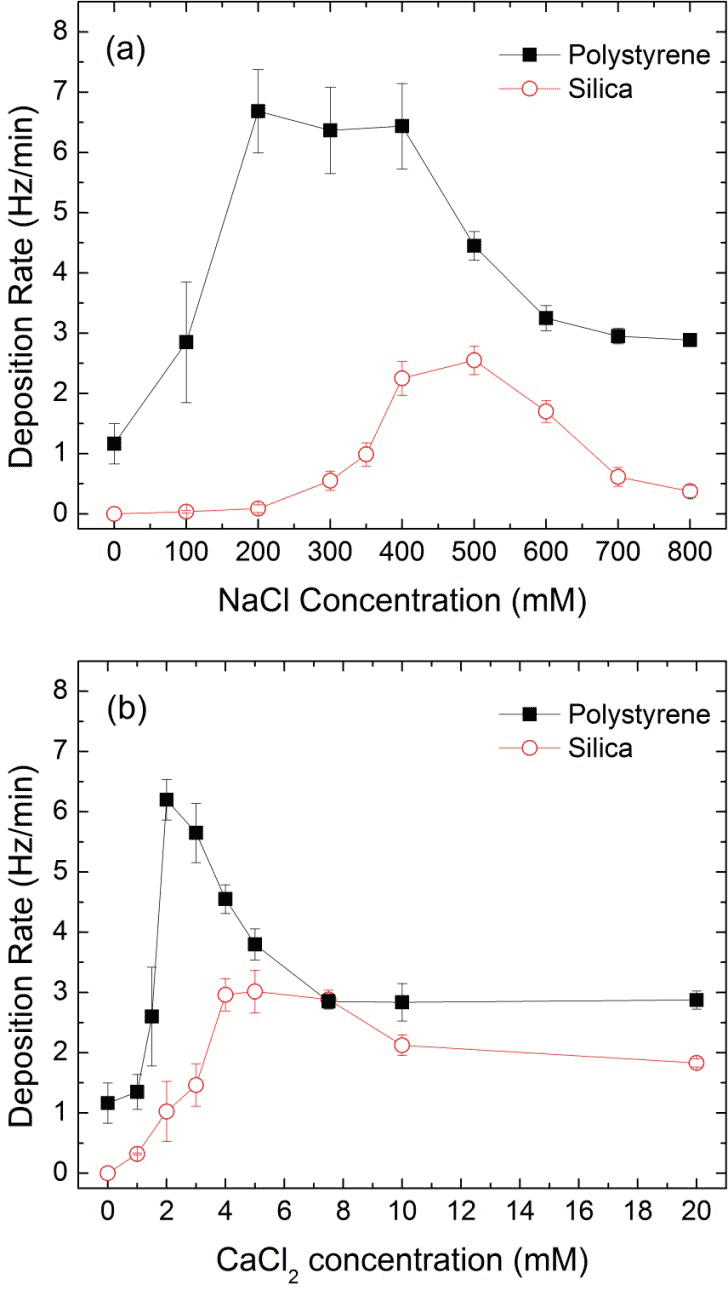
Task 4. Deposition Kinetics. Using a quartz crystal microbalance with dissipation monitoring, oleic acid stabilized iron oxide nanocrystal deposition onto silica (hydrophilic) and polystyrene (hydrophobic) surfaces was evaluated as function of particle size, concentration and solution ionic strength. Generally, deposition rates increased (as measured by the change in frequency over time) as the electrolyte concentrations increased, eventually reaching a maximum. Further increases in electrolyte concentrations cause diffusion-limited transportation, and the deposition rate will decrease. From Figure 5, 8 nm nanocrystals (at 7.5 mg/L, pH 7.2) reach a maximum deposition rate on silica around 500 mM NaCl, while on the polystyrene surface; the fastest deposition concentration for NaCl is between 200-400 mM. The separate peaks in the deposition kinetics of silica and polystyrene surfaces indicate the higher affinity of these nanocrystals onto polystyrene (hydrophobic) surfaces. The most favorable deposition concentration in the presence of NaCl is significantly higher than that in the presence of CaCl2. Further, deposition energy dissipation measurements further support these results indicating significantly increased deposit(s) (particle layer) rigidity in the presence of CaCl2 and onto polystyrene surfaces. Similar experiments are ongoing with selected, brine stable core materials
Overall, this project is on schedule – Project Year 2 and will focus on Tasks 3., 5., and 6., in addition to wrapping up Tasks 2. and 4.
Figure 1. TEM micrographs of nanoscale, monodispersed magnetite (Fe3O4) particles synthesized.
Figure 2. Top left TEM images of MnOx-FeOx ferrite nanoparticles with varying Fe:Mn ratios. Bottom left graphs demonstrate size and molar ratio control. FEG-TEM micrograph images on the right (surface) map iron+manganese and demonstrate the mixed materials.
Figure 3. Magnetite nanoparticles can be effectively hollowed (visually confirmed by the 'donut' like appearance in TEM micrograph on right) using trioctylphosphine oxide (TOPO).
Figure 4. Attachment efficiencies (particle stabilities) of 8 nm oleic acid bilayer coated iron oxide nanocrystals as functions of (a) NaCl and (b) CaCl2 concentrations at pH 7.2.
Figure 5. QCM-D deposition rates of oleic acid bilayer coated iron oxide nanocrystals in the presence of (a) NaCl and (b) CaCl2 onto silica and polystyrene surfaces.
Copyright © 2014 American Chemical Society