58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1051312-ND10: Electrochemistry, Surface Chemistry, and Luminescence of Single Fluorophore
Philippe Guyot-Sionnest, University of Chicago
The end goal of the PRF grant is to explore and develop fluorescent probes of the local electrochemical potential. The system chosen is semiconductor nanocrystals because of the demonstrated sensitivity of nanocrystals to injected charges, their robust fluorescence and the possibility of reversibly going over an indefinite number of redox cycles, which are altogether a unique combination of properties. Specifically, the aim is to obtain dots that remain bright in different oxidation states, and that also remain small so that they can be incorporated eventually in biological systems or others. The challenge is that, just as for small molecules, nanocrystals are typically dark when reduced or oxidized. Even in the absence of surface defects that could be created by harsh redox chemistry, the simple presence of an extra mobile carrier in a quantum dot is well known to lead to a fast nonradiative recombination of any photoexcited dot, in effectively a three body collision. This quenching process is called Auger, in analogy to the similar atomic process, and it is an impediment to many electro-optical applications of the quantum dots. The initial goal of the PRF grant was then to understand and control this Auger process, possibly eliminating it.
The bulk semiconductor literature suggested that negatively charged nanocrystals with small electron kinetic energies, either because of reduced confinement at large sizes, or alloying, would exhibit a slower Auger processes, allowing to retain luminescence in charged states and the sensitivity of the PL to the presence of extra charges. The chemical stability of CdSe quantum dot to the injection of negative charge made it an ideal system to study the negative charged II-VI quantum dots. Specifically, the experiments measured the photoluminescence of the negative charged quantum dots, which are in a state called a negative trion. A trion is the name used to describe an exciton in a charged dot, a three particle construct, made of two electrons and one hole for the negative charged dot.
Over the past two years the grant supported one Graduate Student, Wei Qin, who has achieved exceptional results and will soon have three papers published in ACS Nano, papers that all acknowledge the PRF grant. The first paper is “CdSeS/ZnS Alloyed Nanocrystal Lifetime and Blinking Studies under Electrochemical Control” published in ACS Nano in January 2012 and with 12 citations according to the Web of Knowledge. The second paper, “Evidence for the Role of Holes in the Blinking: Negative and Oxidized CdSe/CdS Dots” was published in ACS Nano in October 2012 with already 9 citations. These first two works showed how electrochemical charge injection could be used to modify the blinking of the quantum dots, in particular allowed to suppress the blinking in dots with one extra negative electron. This is attributed to a cathodic protection, as the extra electron prevents oxidation by the photoexcited hole. These works also showed that the photoluminescence of the charged dots, either the alloy CdSeS or the core/shell CdSe/CdS with a thin shell was weak, as expected from prior studies, but not as weak as in pure CdSe. These were encouraging but not definitive indications that reduced confinements was the way to bright charged dots.
This past year, further experiments provided a striking confirmation of this initial intuition, with a study of CdTe/CdSe core/shell quantum dots under electrochemical control. This work is under submission at ACS nano, and likely to be accepted shortly. The main finding is that reducing the kinetic energy of the electron in the region where it has a spatial overlap with the hole is indeed the solution to obtaining long-lived trions. In other words, the collision between the two low kinetic energy electrons is not effective enough to facilitate the electron hole recombination.
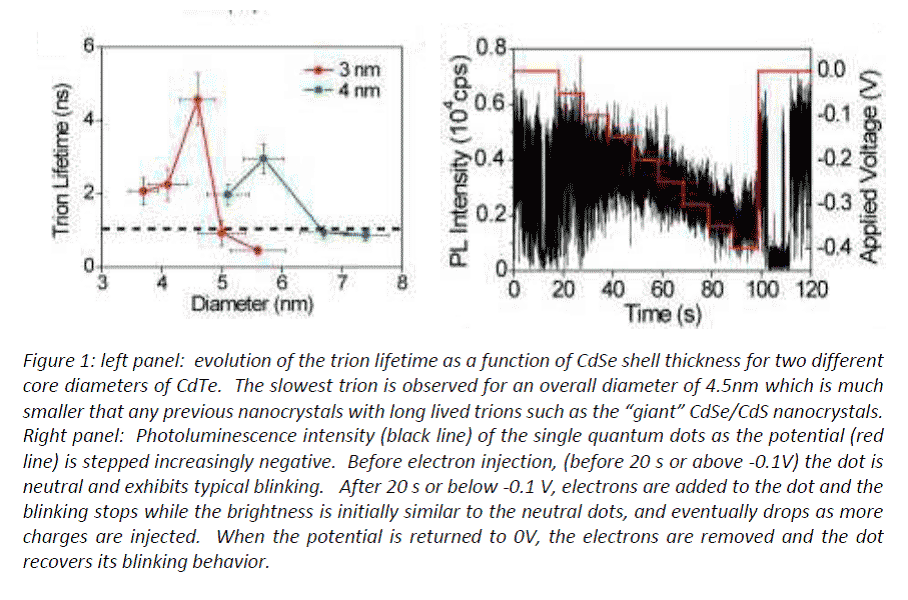
In the CdTe/CdSe core shell systems, the kinetic energy of the electron could be tuned over a wide range with the CdSe shell on the CdTe core. For a particular shell thickness, it is expected that the electron in the CdTe core can have a zero kinetic energy, lying right at the bottom of the CdTe conduction band.
Wei found indeed that the trion lifetime and brightness was maximum for a specific shell thickness and that this shell thickness was consistent with the zero kinetic energy argument. The left panel of Figure 1 shows the maximum of the trion lifetimes measured over an ensemble of CdTe/CdSe quantum dots as a function of shell thickness. The right panel of Figure 1 shows the effect of the charging on the brightness and blinking of a single CdTe/CdSe dot within the sample exhibiting the longest trion lifetime.
In summary, in the second year of the PRF grant, a significant result was obtained allowing ot better understand and control the carrier induced recombination in quantum dots. Small bright charged dots, postulated at the stage of the proposal, have become a reality in the lab. The experiment however unveiled other aspects that are not yet understood, such as a striking discrepancy between the lifetimes of the negative trion (2 electrons and one hole) and the biexciton (2 electrons and 2 hole) and their trends as a function of shell thickness. This will have to be the subject of further investigations.
In the coming year, the remaining funds will be used to carry out the low temperature study of the charged quantum dots, as detailed in the original proposal. This might allow to control the charge state of the colloidal dots in a low temperature solid state device so that spectroscopy can be performed with high resolution on the trion state.
Within the broader context of chemistry, the successful development of bright charged dots might then lead to local probes of electrochemical potential which will further impact the study of nanostructured materials, with potential applications for corrosion studies, electrocatalysis or fuel cells, while for electrooptical applications of the quantum dot materials, bright charged dots might lead to more efficient electrically driven light emitters, and possibly lasers.
Copyright © 2014 American Chemical Society