58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI551459-DNI5: Engineering Interfacial Properties of Emulsions to Limit Transport of Free Radicals and Oxygen Across the Interface
Nitin Nitin, PhD, University of California (Davis)
Progress Report
Aug 2012-Aug 2013
During the last year, we have focused our research efforts on engineering the physical and chemical barrier properties of the interface in improving oxidative stability of emulsions.
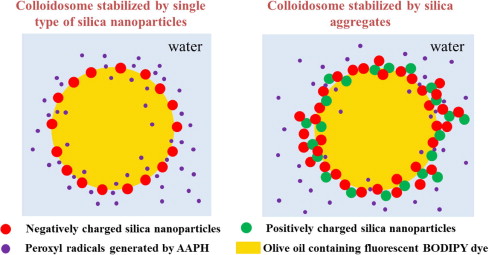
Fig. 1: Engineering the physical barrier properties of particle stabilized emulsion |
(a) Self-Assembly of Silica Clusters at the interface to improve oxidative barrier properties
Although silica based particles have been used to stabilize emulsion but due to electrostatic repulsion between similarly charged particles, shells composed of single-type nanoparticles tend to be monolayer-thick and relatively permeable. We investigated a self-assembly method for controlling the permeability of colloidal shells using aggregates composed of oppositely charged silica nanoparticles (Figure 1).
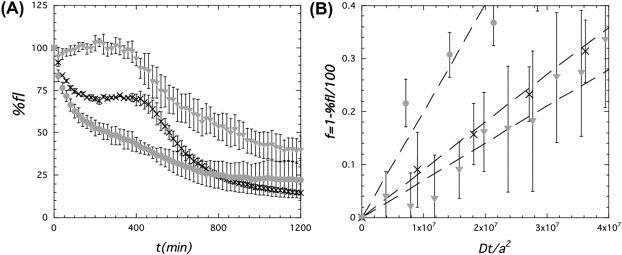
Fig. 2: Effect of the barrier properties of the interface on the rate of permeation of free radicals |
Using a combination of rapid fluorescence based method and theoretical diffusion models, we found that colloidosomes whose shells contained colloidal silica aggregates displayed lower permeability to peroxyl radicals than ones stabilized by single type of silica nanoparticles (Figure 2). Furthermore, the permeability varied as a function of the ratio of oppositely charged silica nanoparticles in the shell. The ability to control the permeability of colloidosomes, while using a simple self-assembly synthesis method, will enable enhanced control over release kinetics and oxidative stability of encapsulants.
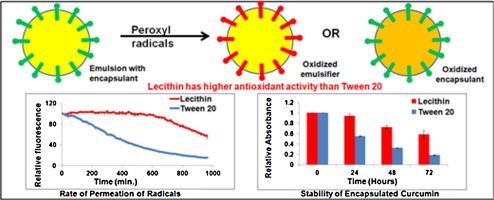
(b) Effect of antioxidant properties of emulsifiers on oxidative stability of encapsulated compounds in emulsions
The objective of this study was to evaluate the role of antioxidant properties of common emulsifiers (lecithin and Tween 20) in reducing permeation of free radicals across the emulsion interface (Figure 3).
Radical permeation rates were correlated with oxidative stability of a model bioactive compound (curcumin) encapsulated in these emulsions. Rate of permeation of peroxyl radicals from the aqueous phase to the oil phase of emulsion was inversely proportional to the antioxidant properties of emulsifiers. The rate of radical permeation was significantly higher (p < 0.05) for emulsions stabilized using Tween 20 and oxidized lecithin compared to native lecithin that showed higher antioxidant activity. Free radical permeation rate correlated with stability of curcumin in emulsions and was significantly higher (p < 0.05) in lecithin stabilized emulsions as compared to Tween 20 emulsions. Overall, this study demonstrates that antioxidant activity of emulsifiers significantly influences permeation of free radicals across the emulsion interface and the rate of oxidation of bioactive encapsulant.
Advancing career of my students and my laboratory: The ACS support of this research was instrumental in advancing training of a postdoctoral fellow and a graduate student (not directly funded by ACS) and developing collaboration with researchers at Drexel University. Based on the success with these studies, I have submitted my NSF-CAREER proposal and also a NIH-R21 grant application.
Copyright © 2014 American Chemical Society