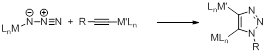58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND351715-ND3: A New Method to Link Metal Ions via Cycloaddition of Metal-Azides to Metal-Acetylides
Adam Veige, University of Florida
Our discovery is the reaction between a metal-azide and a metal-acetylide, given the moniker “iClick” (Scheme 1). Extension of this reaction class will provide a new method for linking two or more metal ions between electronically delocalized units. The applications to follow will be as broad as, or potentially broader than, the original Huisgen cycloaddition considering a large number of combinations of metal ions, ligands, coordination geometries, oxidation states, and redox combinations are plausible for this reaction.
Scheme 1. iClick between a M-azide and M-acetylide.
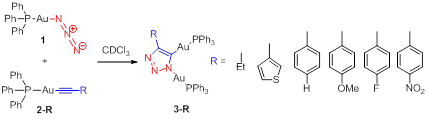
Progress Results 1: Functional group tolerance. Anticipating a need to manipulate the electronic structure of materials derived from iClick reactions, we explored acetylide R-group tolerance and the nature of the ancillary ligand (PR3). Over the past year we have significantly advanced the scope of the AuI/AuI iClick reaction. Scheme 2 depicts the successful reactions now within our repertoire. Clearly, we have firmly established a synthetic protocol for the synthesis of digold complexes linked via various substituted 1,2,3-triazolate bridges.
Scheme 2. iClick between AuI-azide (1)/AuI-acetylide (2); variation in acetylide R-groups and the X-ray structure of 3-NO2.
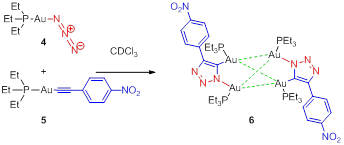
Progress Results 2: Consequences of the ancillary ligand. Our straightforward synthetic studies have uncovered an additional preliminary result that relates to the PR3 group and its impact on iClick chemistry. Changing the PPh3 ligands to the less sterically bulky PEt3 ligand results in an iClick product comprising a C2-symmetric tetragold complex (6) (Figure 1). Figure 1 depicts the synthesis and molecular structure of 6, as determined by a single crystal X-ray diffraction experiment. The tetranuclear metal cluster comprises four gold (I) centers arranged in slightly distorted tetrahedral geometry.
Figure 1. iClick synthesis of Au-tetramer (6) illustrating the consequence of switching PPh3 to PEt3.
Of the relatively few pseudo-tetrahedral gold clusters reported in the literature, this is the first example of a cluster supported by triazole ligands, is one of few examples synthesized in high yields, is a neutral species, and includes all gold species in the +1 oxidation state. This bright yellow material also exhibits fluorescence, with an absorbance at 405 nm and an emission at 539 nm. Further studies of the optical properties of 6 will continue. The formation of 6 highlights the effect that the steric bulk of the ancillary ligands can have in the gold(I) iClick reaction.
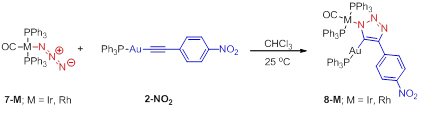
Progress Result 3: iClick synthesis of traizolate linked AuI/IrI and AuI/RhI complexes. The potential synthetic power of iClick is dependent on the scope of metal ions amenable to the reaction conditions. Metal-azides and metal-acetylides are known for nearly every transition metal ion in the periodic table. The sheer number of possible combinations, especially if variations in oxidation state and choice of ancillary ligand are taken into consideration, is staggering. Treating the trans-azidocarbonylbis(triphenylphosphine)iridium(I) 7-Ir and trans-azidocarbonylbis(triphenylphosphine)rhodium(I) 7-Rh with triphenylphosphinegold(I)-para-nitrophenyl-acetylene, 2-NO2 , in CDCl3 results in the formation of the heterobimetallic triazolate bridge IrI/AuI (8-Ir) and RhI/AuI (8-Rh) complexes, respectively. In the iridium case for example, inspection of the 31P NMR spectra as a function of time, reveals that resonances attributable to the phosphine ligands in the starting materials quickly disappear, concomitant with the appearance of two new resonances (23.79 and 43.99 ppm), assigned to the phosphine ligands in 8-Ir. The 1H NMR spectrum of the resulting dark red CDCl3 solution also supports the assignment of complex 8-Ir; the emergence of a characteristic downfield doublet at 7.93 ppm is consistent with the ortho-protons of the nitrophenyl substituent on position 4 of the triazolato bridge.
Scheme 3. iClick reaction between MI-azide (7-M; M = Ir and Rh) and Au-acetylide (2-NO2).
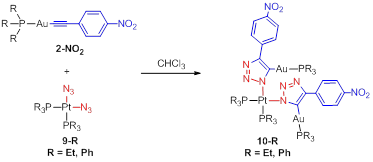
Progress Result 4: iClick synthesis of trimetallic PtII/AuI2 complexes. In attempts to extend the scope of the iClick reaction methodology in the direction of metallopolymers the platinum-diazido complexes 9-R (R = Et, Ph) were synthesized. When paired with metal centers containing multiple acetylide groups, a metallopolymer can form. As a proof of their competence in the iClick reaction however, we coupled the platinum-diazidos (9-R, R = Et, Ph) to the Au-acetylide complex 2-NO2, according to Scheme 4. An X-ray diffraction experiment performed on single crystals of both 10-R complexes provides unequivocal evidence for their molecular structures as trimetallic complexes. The products are the result of a double iClick reaction 10-Ph (left) and 10-Et (right).
Scheme 4. iClick synthesis of a trinuclear PtII/AuI2 complexes 10-R (R = Et, Ph).
Copyright © 2014 American Chemical Society