58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND551448-ND5: Novel Mixed Metal Carbide Catalysts for Petroleum Processing
Friederike C. Jentoft, Dr. rer. nat., University of Oklahoma
Introduction
The goal of this research project is to tune the catalytic activity of carbides by combining two transition metals in a single phase carbide. To reach this goal and to establish structure–activity relationships, we are developing methods for the synthesis of mixed metal carbides with high surface area, are characterizing the obtained carbides, and are testing them for catalytic reactions requiring a metal or an acid–base function.
Traditional methods to synthesize carbides require high temperatures and thus lead to sintering and low surface areas. Preparations of high surface area carbides generally follow a two-step procedure, consisting of the manufacture of a suitable precursor and the subsequent carburization in reducing atmosphere (with, e.g., a hydrocarbon as carbon source). In 2012, we reported on two methods to arrive at mixed metal carbides, one characterized by a co-precipitation step to make the precursor, the other by the addition of hexamethylenetetramine to the precursor, which serves as a reducing agent and as a carbon source (Chouzier et al. 2006). Both methods have their limitations: the first will be unsuccessful if precipitation requires largely different pH values for the two metals in question, the second tends to result in materials containing excess carbon. We have thus explored additional pathways in the past year. Another issue that we have addressed is surface passivation.
Carbides are refractory and have potential as catalysts for high-temperature processing of heavy petroleum components such as polycondensed aromatic molecules. We are using toluene as a model compound to test for two catalytic functions, those of hydrogenation and ring-opening.
Preparation of mixed metal carbides
All of our mixed carbides contained either molybdenum or tungsten as one of the metals; the catalytic activity of Mo2C and W2C for hydrogenation reactions is well documented (Sinfelt and Yates 1971, Levy and Boudart 1973), and these carbides can serve as a starting point and benchmark.
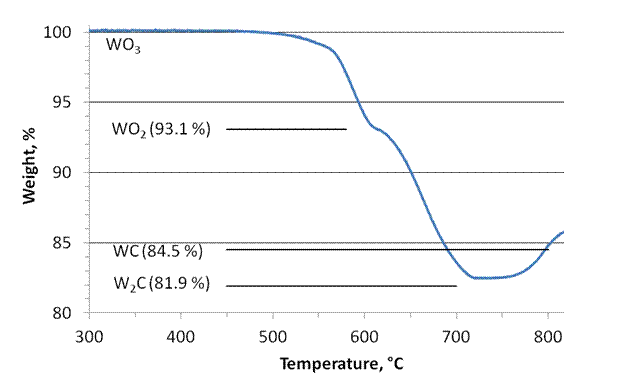
Because we experienced difficulties with adjusting the carbon content when using hexamethylenetetramine-containing precursors we decided to strictly rely on carburization via the gas phase, with hydrocarbons (ethane) as carbon source and H2 as additional reducing agent. An example for the utility of this method to determine the correct carburization temperature needed to produce a specific structure without the addition of extra surface carbon is given is Figure 1, which shows a thermogravimetric analysis of the synthesis of tungsten carbides. WO3 is reduced to WO2, which is transformed into W2C. Further reaction at higher temperatures produces WC as a more carbon-rich product, but concomitant deposition of carbon on the surface occurs easily. This sequence was confirmed through XRD analysis of the individual stages. A fundamental investigation into the carburization kinetics was started because knowledge of the according rate laws will allow us to devise optimal carburization routines.
Figure 1. Thermogravimetric measurement of the carburization of WO3 (in 10% ethane, 70% H2, 20% argon).
A new method that was tested for the preparation of precursors with well interspersed metals is hydrothermal synthesis, which has been reported as a route towards mixed transition metal oxides (Murayama et al. 2012). We tested this method for the synthesis of Mo-Nb carbides and succeeded in preparing a series of carbides with lattice parameters that varied linearly with composition (following Vegard's law), indicating that true solid solutions were obtained. Figure 2 shows the lattice parameter a as a function of the niobium content.
Figure 2. Lattice parameter a for Nb-Mo mixed carbides
However, these carbides were not particularly active for toluene hydrogenation. We hypothesize that catalytic activity, which depends on the electronic structure, was absent because the materials crystallized in the structure of NbC (ICCD# 00-038-1364) and not in the structure of Mo2C (ICCD# 01-071-0242). Samples with a higher molybdenum content would thus be desirable but we could not obtain these stoichiometries via hydrothermal synthesis. This method thus has limitations. It should also be noted that product yields were generally low for the element combinations investigated.
Another approach that was explored was the flash-freezing and subsequent freeze-drying of solutions containing two metal salts. This method is reportedly suitable to prepare mixed oxides via low temperature calcination of the freeze-dried solid (Vie et al. 2004). We adapted this method for our purposes by replacing the calcination by a carburization. Mixed Nb-Mo samples with various stoichiometries (including molybdenum-rich) could be produced. The materials were generally extremely fine powders, thus complicating structural analysis.
In conclusion, there may not be a single pathway to mixed carbides, simply because of the variations in aqueous phase chemistry of the respective transition metals. Two promising routes have been identified, and single-phase mixed carbide materials with tunable stoichiometry have been produced.
Investigation of the passivation of carbides
Carbides such as Mo2C are pyrophoric and must be passivated before they can be transferred between apparatus in ambient conditions. The most common procedure reported in the literature is passivation at room temperature in low concentrations of O2 (Oyama et al. 1988), which presumably leads to a thin surface oxide layer. Recently, passivation in CO2 atmosphere was reported as being advantageous because the catalyst is more easily re-reduced to expose the active carbide surface (Wu et al. 2004). We compared passivation in O2 with passivation in CO2 using thermogravimetry to monitor the weight gain resulting from surface oxidation. Our findings indicate that passivation in CO2 is a slow process that is not clearly terminated through a stop in weight gain, even at elevated temperatures.
Impacts on PI and students
The ACS-PRF grant was a cornerstone in the PI's tenure and promotion dossier and allowed her to expand into a new area, thus significantly affecting her career. The graduate student has reported his research on various occasions: two posters (Laurance Reid Gas Conditioning Annual Conference and Southwest Catalysis Society Spring Symposium) and one oral presentation (58th Annual Oklahoma ACS Pentasectional Meeting). One undergraduate student gained experience in solid-state kinetics through fitting thermogravimetric data, another is learning how to synthesize inorganic nanomaterials.
Copyright © 2014 American Chemical Society