58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI550794-DNI5: Intermetallic Base-Metal Catalysts for the Selective Hydrogenation of Acetylene and Multifunctional Organic Compounds
Robert Rioux, PhD, Pennsylvania State University
Catalysts that exhibit chemoselectivity, or the preferential reactivity of one functional group over another, are highly desired in the field of catalysis. An example of a reaction where a catalyst with chemoselectivity is required is acetylene semi-hydrogenation. Preferential reactivity of triple bonds over double bonds allows for trace acetylene to be removed from ethylene feed streams destined for ethylene polymerization. A good catalyst for this reaction converts all of the acetylene to ethylene without further converting any ethylene to ethane. The industrial catalyst that performs this reaction is a palladium-silver alloy which demonstrates high selectivity towards ethylene. We are developing catalysts composed of nickel and zinc, two abundant metals that allow for the creation of low-cost alternatives to palladium-silver. There is little research that examines the catalytic behavior of Ni catalysts that have been modified with Zn. Pure Ni is inherently unselective for ethylene production, instead producing ethane and oligomeric by-products. Oligomers are particularly unfavorable since build-up causes catalyst deactivation. Ni-Zn catalysts with well-defined structures were examined for their selectivity during acetylene semi-hydrogenation in the presence of ethylene. We used isotopic labeling to determine the mechanism of the reaction and the influence of Zn modification on selectivity.
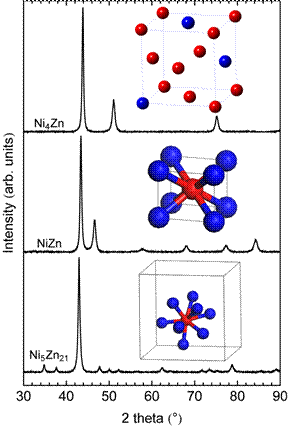
Intermetallic Ni4Zn, NiZn, and Ni5Zn21 were synthesized using a bulk synthesis technique to create phase pure compounds as evidenced by x-ray diffraction (Figure 1).
Figure 1. X-ray diffraction patterns of Ni4Zn, NiZn, and Ni5Zn21 catalysts. All of the observed peaks coincide with the calculated pattern of each structure. Insets show the atoms in the unit cells. Ni4Zn forms a substitutional solid solution with the face-centered cubic structure. NiZn forms the tetragonal L10 structure with each Ni atom coordinated by eight Zn nearest neighbors. Ni5Zn21 adopts the cubic γ-Brass structure with 52 atoms in the unit cell (not all atoms are shown for clarity).
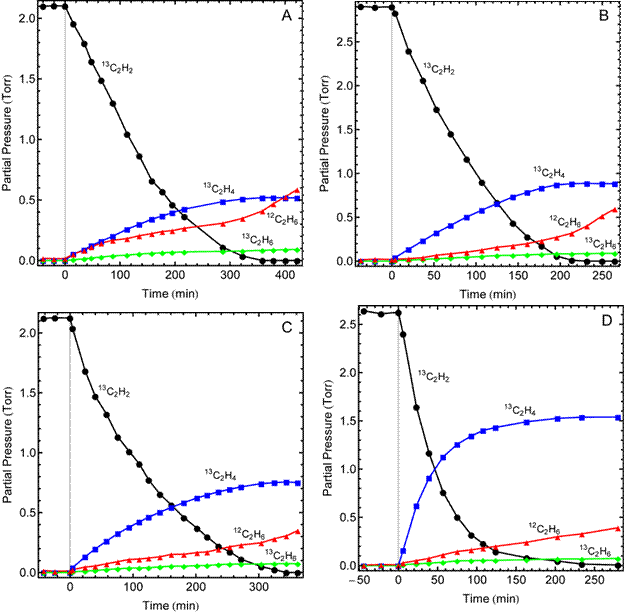
The selectivity for acetylene semi-hydrogenation in the presence of ethylene was measured in a batch reactor with 13C-labeled acetylene and excess ethylene (predominately 12C). Isotopic labeling allows for accurate quantification of the amount of ethylene produced from acetylene. It allows for determination of the origin of ethane since ethane can be produced from either acetylene or ethylene. The catalytic behavior of Ni4Zn, NiZn, and Ni5Zn21 were compared with Ni and the results are shown in Figure 2.
Figure 2. Catalytic behavior for Ni (A), Ni4Zn (B), NiZn (C), and Ni5Zn21 (D) during the hydrogenation of a mixture of 13C2H2 and 12C2H4 at 160 °C in a batch reactor. Initial partial pressures of 13C2H2, H2, and 12C2H4 were ~2.5 Torr, 27 Torr, and 53 Torr, respectively. The first two data points of each data set are at room temperature, prior to heating. The vertical line indicates the point at which the reactor reached 160 °C. Three and four carbon species were also measured but are not shown for clarity.
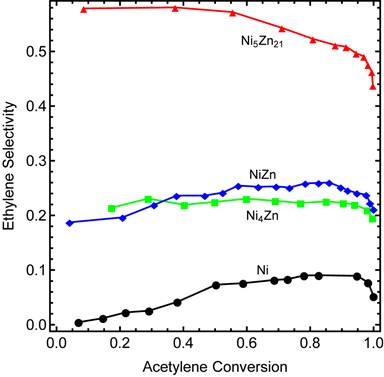
The most important parameter in evaluating the effectiveness of an acetylene semi-hydrogenation catalyst is ethylene selectivity. Ethylene selectivity is defined as the net ethylene production per acetylene removal. The acetylene produced from ethylene is directly measured as the amount of 13C2H4. The gas phase ethylene that reacts to form ethane is measured as the amount of 12C2H6. The net ethylene production is defined as the amount of 13C2H4 formed minus the amount of 12C2H6 formed. Acetylene removal is quantified by taking the difference between the initial and final amounts of acetylene in the reactor. The maximum ethylene selectivity is 1 and ethylene selectivity maybe be negative which corresponds to a net decrease in the amount of ethylene. Figure 3 displays ethylene selectivity as a function of acetylene conversion for each of the Ni-Zn catalysts. As the Zn content increases, ethylene selectivity increases.
Figure 3. Ethylene selectivity during the selective hydrogenation of acetylene in the presence of ethylene for Ni, Ni4Zn, NIZn, and Ni5Zn21. Ethylene selectivity is defined as the net ethylene production per acetylene removal. The inclusion of zinc into nickel results in an increase in the ethylene selectivity.
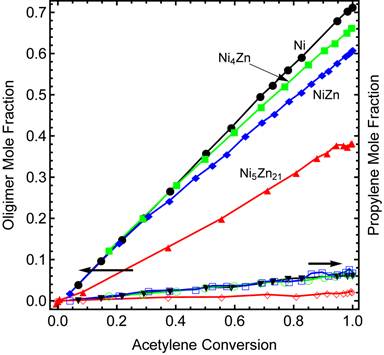
The amount of ethane produced was similar for each of the intermetllic Ni-Zn compounds and pure Ni (Figure 2). The benefit of adding Zn is the reduction in carbon-carbon bond formation. Figure 3 shows the oligomer mole fraction as a function of acetylene conversion for each of the catalysts tested. In Ni5Zn21, Zn geometrically isolates Ni atoms which reduces oligomer formation.
Figure 4. Oligomer mole fraction (three or more carbon species) and propylene mole fractions as a function of acetylene conversion. The primary role of zinc is reducing the formation of oligomeric products.
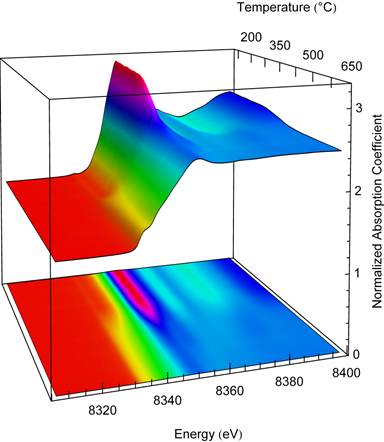
The bulk synthesis technique creates Ni-Zn compounds with low surface area catalysts. Practically, it is necessary to create a high surface-area catalyst so that a high activity may be realized. We used wet impregnation whereby Ni nitrate was added to ZnO and calcined to create NiO nanoparticles supported on ZnO nanoparticles. X-ray absorption near-edge structure was used to probe the reduction of the Ni and intermetallic formation by monitoring the reduction in situ (Figure 5). This demonstrates the feasibility of creating a high surface area Ni-Zn intermetallic catalyst.
Figure 5. X-ray absorption near edge structure of the temperature evolution of the in situ reduction of 2 wt. % Ni/ZnO. NiO nanoparticles are reduced at ~350 °C as evidenced by a decrease in the height of the edge and a shift in energy of the onset of absorption. A Ni-Zn substitutional solid solution forms at ~450 °C and intermetallic NiZn forms at ~600 °C.
This work has had a very positive influence on the graduate student working on this project (Charles Spanjers) due to the challenges that were overcome with regards to creating and characterizing well-defined materials at the nanoscale. He utilized principles from materials science, chemical engineering, and physics to fully understand this system. This work will result in a number of publications. This grant was a catalyst to the PI's independent research career, including be the first of those secured by the PI, including a number of young faculty awards.
Copyright © 2014 American Chemical Society