58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI751618-DNI7: Synthesis of Depolymerizable Polymers
Scott Phillips, PhD, Pennsylvania State University
Introduction. The specific goal of the proposed project was to determine the impact of structural perturbations on each repeating unit on the rate of signal-induced head-to-tail depolymerization of poly(carbamates). The general depolymerization reaction mechanism for these poly(carbamates) is shown in Figure 1. We specifically hypothesized that we could raise the ground state energy of each repeating unit by increasing the electron density of the aromatic ring in each repeating unit, thus decreasing the activation energy required for depolymerization. Similarly, we predicted that if we decreased the aromaticity of each repeating unit, then we could decrease the activation energy for depolymerization and accelerate the rate. This final report describes our observations and conclusions related to both hypotheses.
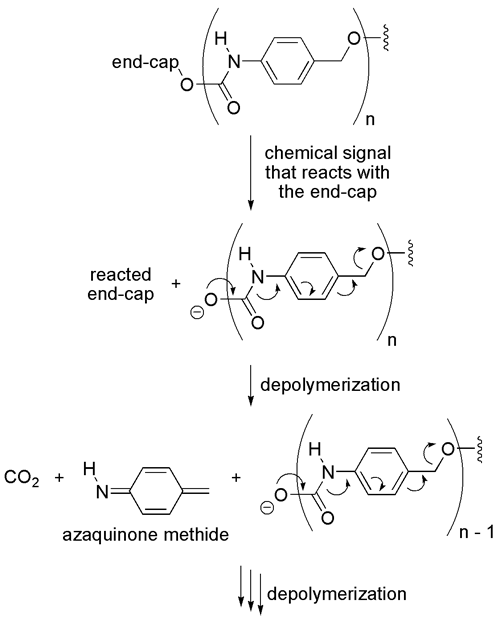 |
Figure 1. Proposed step-wise depolymerization pathway for the poly(carbamates).
Results and Discussion
Hypothesis 1: Effect of electron density on the rate of depolymerization of poly(carbamates). We tested the first hypothesis using a small molecule model system, followed by studies with discrete carbamate oligomers. The small molecule system revealed that placement of electron donating groups (such as methyl ethers) ortho to the benzylic leaving group accelerates the rate of quinone- and azaquinone-methide mediated release of the benzylic leaving group (Figure 2). This result is described in detail in J. Org. Chem. 2012, 77, 4363–4374.
Figure 2. A small molecule model system for measuring the effect of electron donating substituents on the rate of release of phenol when the Alloc group is cleaved in response to Pd(0). The half-life values were measured using HPLC.
Based on these results with the small molecule model system, we prepared discrete carbamate oligomers that depolymerize in response to hydrogen peroxide. The depolymerization rates in the carbamate oligomers are consistent with the small molecule model system, and thus reveal that strategic placement of electron donating groups accelerate the rate of head-to-tail depolymerization of poly(carbamates). The carbamate oligomer work was published in J. Org. Chem. 2013, 78, 3159–3169.
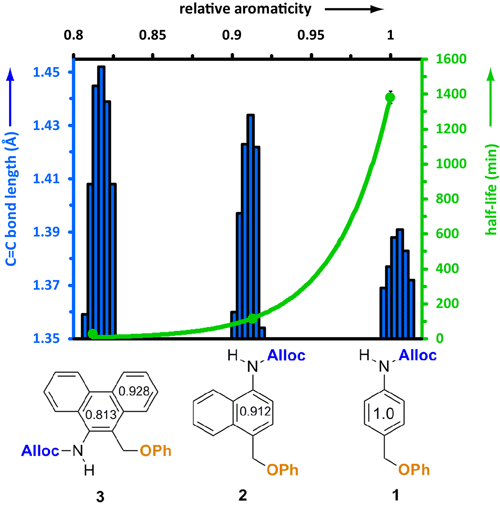
Hypothesis 2: Effect of aromaticity on the rate of depolymerization of poly(carbamates). We tested the second hypotheses using the same work-flow as for the first hypothesis: i.e., we used a small molecule model system first, followed by application of the results in the context of carbamate oligomers. The small molecule model system demonstrated that faster rates of release of phenol (via azaquinone methide) could be realized as the aromaticity of the release reagent decreased (Figure 3). This work is detailed in J. Phys. Org. Chem. 2013, 26, 608–610.
Figure 3. Effect of aromaticity on the rate of release of phenol when the Alloc group is cleaved in response to Pd(0).
We subsequently applied the observations from Figure 3 in the context of depolymerizable carbamate oligomers and compared the effects of aromaticity with the effects of electron donating groups on the rate of depolymerization (Figure 4). This comparison was published in J. Org. Chem. 2013, 78, 3159–3169.
Figure 4. Effect of aromaticity and electron density on the rate of depolymerization of poly(carbamates).
Concluding remarks. We successfully completed the studies in our proposed work and concluded that the rate of head-to-tail depolymerization of poly(carbamates) can be accelerated substantially by appropriately modifying each repeating unit in the polymer. In the case of the current studies, we observed rate accelerations of at least 143×.
Future work will continue to evaluate the effects of structural modifications on the rate of depolymerization of poly(carbamates). Depolymerizable poly(carbamates) represent an interesting class of compounds that are derived from petroleum resources. Many applications of these polymers are envisaged, but faster rates of depolymerization are required before the applications can be realized. As such, this ACS-DNI funded work has pointed us in a promising direction for improving the response properties of these polymers via careful physical organic studies. This work has spawned several projects in my group to continue this line of investigation, and has provided the basis for three publications and one Ph.D. thesis.
Two graduate students and one undergraduate contributed to the results outlined in this final report, and three additional graduate students have contributed partial effort to extending these results into new systems. One graduate student has obtained his Ph.D. based predominantly on these studies, while another is close to graduating with a Ph.D. by using the results of these studies for more applied endeavors. The undergraduate student is now employed at DuPont.
Copyright © 2014 American Chemical Society