58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI552422-DNI5: Design of Zeolite Growth Modifiers: A Hierarchical Approach to Optimize Nanoporous Catalysts
Jeffrey D. Rimer, PhD, University of Houston
The goal of this project is to develop rational approaches in zeolite synthesis to selectively tailor material properties for improved performance in catalytic applications. The specific objectives targeted for this period included the following: (i) Systematic investigation of zeolite growth modifiers (ZGMs) with varying site specificity for growing surfaces of zeolite L (LTL type) crystals; (ii) Optimization of synthesis parameters as a comprehensive method for tailoring zeolite crystallization in concert with the design of effective ZGMs; and (iii) Developing novel analytical tools in zeolite surface science capable of characterizing the effects of ZGMs on surface growth.
Modification of Zeolite LTL Crystallization. Crystals of zeolite LTL consist of large 1-dimensional (1D) pores and a cylinder morphology with pore channels oriented along the axial direction (see Figure 1). LTL was selected as a candidate for habit modification based on its severe mass transport limitations (i.e. long diffusion path length and small porous surface area). As part of this project, we explored the use ZGMs, which are inexpensive, readily-available commercial organics selected with site-specificity for binding to preferential crystallographic faces of zeolites and modifying anisotropic rates of growth. The ZGMs selected for LTL experiments were primarily polyols. Scanning electron microscopy (SEM) was used to analyze the effects of each ZGM on the bulk crystal habit. Our studies revealed ZGMs with site specificity for the (001) face, which reduces growth along the axial direction to produce thin platelets (see Figure 1, b and d). We observed clear trends in ZGM efficacy that provided heuristic guidelines for the selection of effective growth modifiers of (001) faces. For instance, the ZGM 1,2,3-hexanetriol had the most pronounced effect on crystal habit, producing thin [001] platelets with diffusion path lengths equal to ca. 100 nm while simultaneously preserving large porous (001) surface area (e.g. 1 mm2/particle). We used atomic force microscopy (AFM) to provide microscopic validation of ZGM interactions with LTL crystal surfaces, showing that we can produce either smooth or highly corrugated surfaces depending on the judicious selection of ZGMs.
Figure 1. (a and c) Zeolite LTL crystals grown in the absence of modifiers. (b and d) ZGMs with site specificity for (001) faces of LTL crystals produce thin platelets. (e) The crystal structure of LTL consists of 1-dimensional channels oriented in the [001] direction.
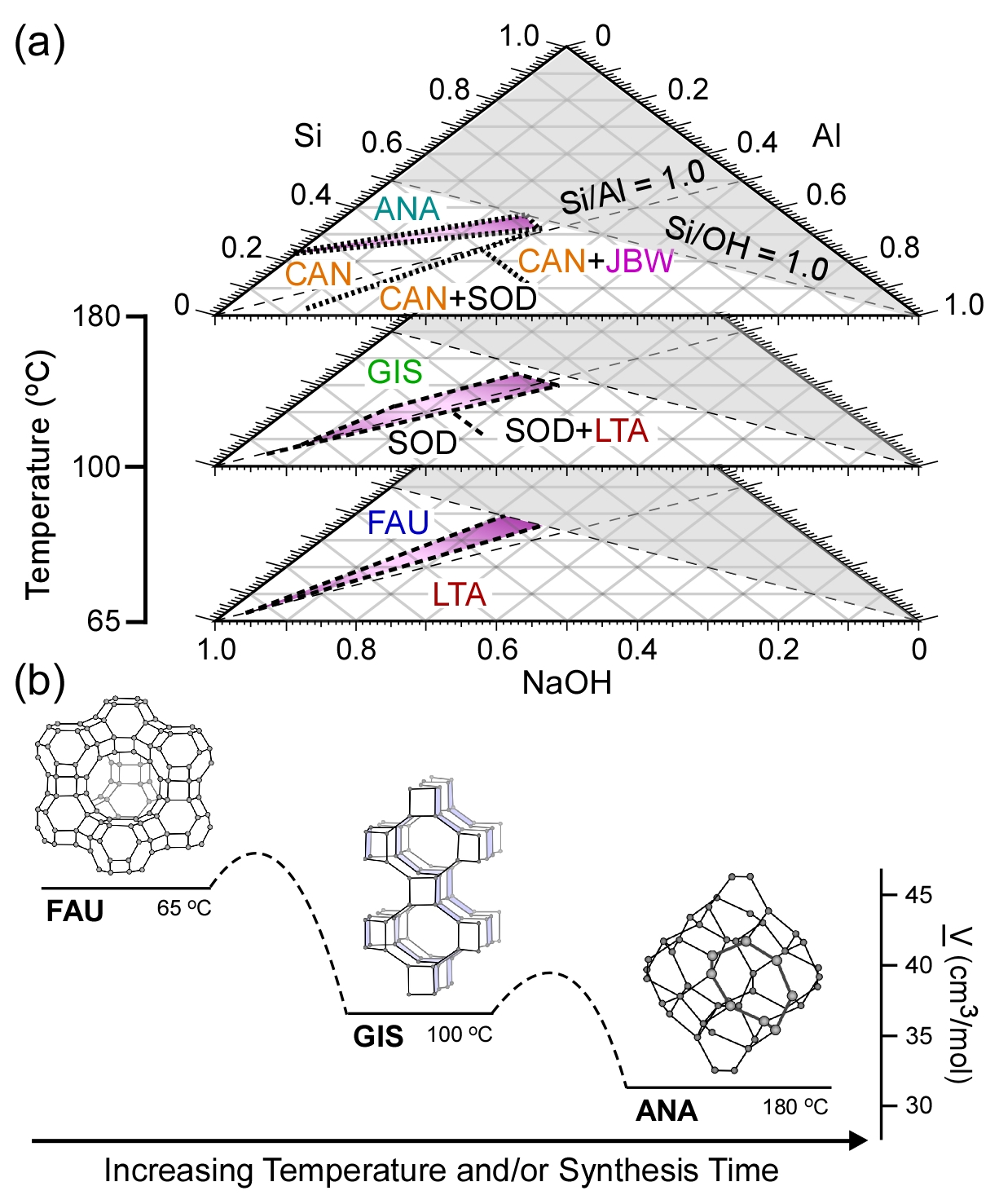
New Platforms to Optimize Zeolite Synthesis. In conjunction with research efforts to identify and test ZGMs, we have also begun to search for heuristic methods that permit a priori determination of growth solution compositions to tune the physicochemical properties of zeolite catalysts, while simultaneously minimizing the formation of crystal polymorphs. Efforts in the first period of this project have focused on the generation of kinetic phase diagrams (see Figure 2), which are ternary plots of the three principal reagents: SiO2, Al2O3, and Na2O. These diagrams reveal the changes in crystal phase behavior at various synthesis temperatures where the complexity of the phase space clearly increases with temperature, resulting in regions of pure crystals as well as multiphase regions. The transformations in crystal structures that we observed generally abide by Ostwald's rule of stages, which predicts transformations from an initial metastable structure (high molar volume) to more thermodynamically stable structures (low molar volume), as shown in Figure 2b. We are in the process of combining these efforts with ZGM design to collectively tailor zeolite properties, such as crystal composition, size, and morphology.
Figure 2. (a) Kinetic phase diagrams for zeolites synthesized at various temperatures. The three letter codes are in reference to different crystal structures. The results of these studies are discussed in a recent review article (Oleksiak and Rimer, Rev. Chem. Eng., 2013). (b) Transformations in crystal structure with increased temperature and/or synthesis time generally abide by the Ostwald rule of stages, which predicts changes from an initial metastable phase to structures that are progressively more thermodynamically stable.
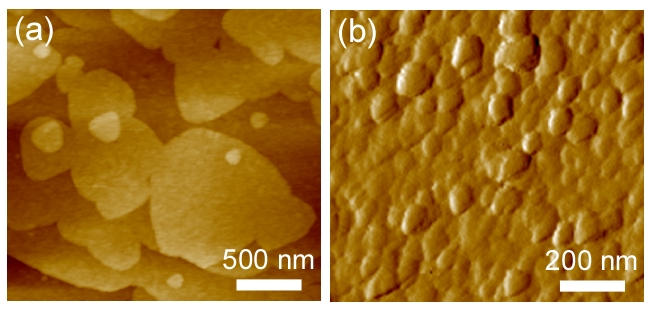
AFM Measurements of Zeolite Surface Growth. A percentage of time spent on this project was devoted to the design and testing of an AFM sample cell that was retrofitted for in situ imaging under conditions amenable to zeolite synthesis. Our initial testing of this system focused on the growth of silicalite-1 (siliceous MFI type). We prepared silicalite-1 crystals with large (010) surfaces that were amenable for AFM analysis. The crystals were mounted in epoxy with their surfaces oriented in the plane of analysis. Using a closed liquid cell equipped with inlet/outlet ports for growth solution supply, we continuously imaged silicalite-1 (010) surfaces in supersaturated growth solutions (pH 10 – 13) at a temperature of 80 ºC for timescales of 10 to 48 hours. The results of these studies provided valuable information regarding silicalite-1 growth mechanisms (see Figure 3), and permitted the quantification of anisotropic growth rates in different crystallographic directions. We are currently using this setup to analyze a range of synthesis conditions, and to demonstrate the broader applicability of this approach in zeolite surface science.
Figure 3. Representative AFM deflection mode images during the in situ growth of a silicalite-1 (010) surface under realistic synthesis conditions (i.e. pH 11, 80 ºC, and supersaturated silica). (a) The initial substrates used for these studies were comprised of single layer steps (ca. 0.7 nm in height) corresponding to pentasil layers of the MFI crystal structure. Terraces of these substrate layers are relatively smooth. (b) An image of the same surface taken after nearly 10 hours of continuous imaging in growth solution. The results of these studies reveal concerted processes of classical and non-classical crystal growth by the formation and growth of 3D islands.
This award has partially supported the research of one graduate student, one undergraduate student, and one postdoctoral researcher. The results of this period have resulted in two published manuscripts and a third that is currently in review. Moreover, students and the PI have presented this work at conferences and seminars, including the American Chemical Society Spring and Fall Meetings, the American Institute of Chemical Engineering Annual Meeting, and the Gordon Research Conference on Nanoporous Materials and Their Applications.
Copyright © 2014 American Chemical Society