58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI1050237-UNI10: Controlling Morphology and Electronic Properties of Two-Dimensional Organometallic Conjugated Polymers via Orthogonal Polymerization Methods
Katsu Ogawa, Ph.D., California State University (Northridge)
Although the number of reports on new phosphole based π-conjugated systems is increasing each year, use of phosphole in optoelectronic devices is still not as common as thiophene or pyrrole based analogues. The major problems seem to be the limited synthetic accessibility of the phosphole moiety and the stability of the resulting phosphole based compounds. One of our research goals is development of convenient synthetic routes for phosphole based oligomers as π-conjugated cross linkers for 2D conjugated polymer matrix to improve charge migration efficiency. In our approach, phosphole type ligands are incorporated in place of typical trialkyl phosphine ligands for platinum acetylide polymers. Unlike its nitrogen analogue (pyrrole), the lone pair on phosphorus atom is not completely associated with the π system of the phosphole ring. As a result, phosphole exhibit non-planar structure with ligating capability through the lone pair. On the other hand, phospholes can be polymerized via oxidation similar to pyrroles and thiophenes. The introduction of phosphole ligands in Pt-acetylide polymers results in possibility for cross linking a conjugated polymer (Pt-acetylide) with another conjugated polymer (Polyphosphole). Such cross linking should lead to increased conjugation, hence the improved charge migration for enhanced efficiency of the optoelectronic devices.
Accomplishments in the First Two Years
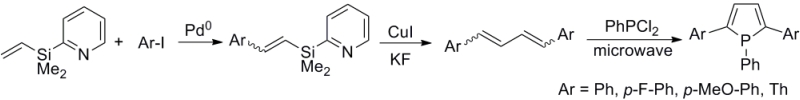
A synthetic route to 1-phenyl-2,5-bis(2-thienyl)phosphole was developed using 1,4-bis(2-thienyl)butadiene as a precursor as shown below. Although each step gave moderate yields (60-80%), the overall yield of the butadiene was less than 5 % due to the number of steps involved. Initial attempts to synthesize 1-phenyl-2,5-bis(2-thienyl)phosphole ligand resulted in very low yields (2~3%). Major byproduct was insoluble polymeric material that is most likely due to polymerization of the precursor diene and/or the desired product. Another major byproduct seem to be a p-p linked dimer, which is inseparable from the desired product by conventional separation technique such as column chromatography. The effect of the temperature on byproduct formation has been investigated extensively. Above 160 °C, dimer formation is favored. Below 150 °C, dimer formation is negligible, however, the conversion of the starting material to the product is very slow that the reaction requires up to 5 days to complete. Although dimer formation can be suppressed by lowering the temperature, the amount of polymeric byproduct increased due to prolonged reaction time. The optimal condition gave only 14% yield of pure desired product.

Photophysical and electrochemical characterizations have been performed on both the precursor 1,4-bis(2-thienyl)butadiene and the ligand 1-phenyl-2,5-bis(2-thienyl)phosphole. Bathochromic shifts of absorption (~60 nm) and emission (~70 nm) maxima are observed upon annulation of butadiene moiety by introduction of phosphorus atom. Extended π system results in lower HOMO-LUMO energy gap. Oxidation potential of the ligand (+741 mV) is higher than that of the precursor (+588 mV). Upon cycling of the potentials, the precursor molecule can be successfully polymerized as expected for thiophene derivatives. The ligand, however, the oxidation occurs at the phosphorus instead of the sulfur on thiophene resulting in decomposition. Similar phenomena have been observed for other phosphole containing oligomers.
Accomplishments in the Third Year
A new synthetic scheme has been utilized to improve the overall yields and to reduce the number of steps from commercially available compounds. Several precursor bis(aryl)butadienes were synthesized following a literature procedure. This new scheme allows incorporation of various aryl groups more easily than the previous synthetic scheme. This year's major improvement for our project was the use of microwave assisted syntheses. Instead of utilizing conventional heating methods, the annulation was accomplished by microwave heating of the reaction mixture. Conventional heating tends to give higher temperature at the contact surface and lower core temperature for the reaction mixture. The microwave heating provide the opposite heating profile with higher core temperature with lower temperature at the glassware surface. This inverted heating profile minimized the formation of byproducts at the higher temperature surface while keeping the core temperature above optimal reaction temperature for the product formation. The use microwave significantly improved the product yields and shorten the reaction time from 5 days to 3 hours. Not only did it improved the yields but also resulted in better purity by suppressing both the dimer and polymer formation.
The Impact of the ACS PRF UNI Grant on the PI and Students
The UNI grant provided essential financial support for PI's research activities. The continuation of the research would have been impossible without UNI grant after the start up funding from the institution was exhausted for purchasing spectroscopic analytical instrumentations. Total of three undergraduate students were actively involved in the project during last year. Two of these students are still active in this project. One of them has become a graduate student.
Plans for the Fourth Year
The priority is given to preparation of platinum metal complexes. The phosphole ligands will be treated with K2PtCl4 to obtain a platinum complex bearing chloride ligands and phosphole based ligands. Then photophysical and electrochemical characterization of the complex will be performed. This complex will be chemically polymerized via Hagihara coupling using chloride ligands on the platinum in the presence of diethynylbenzene. The resulting Pt-acetylide polymer will be spin-coated onto an ITO electrode and attempts will be made to cross link the polymer via electrochemical oxidation. The prepared 2D polymer matrix will be characterized by spectroscopic and electrochemical techniques. The preparation and characterization of platinum complex should be accomplished in Fall 2013. Characterization of polymeric materials are to be accomplished in Spring and Summer 2014.
Copyright © 2014 American Chemical Society











