58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051865-DNI10: Understanding Ionic Liquid Aided Graphene Production by Exfoliation of Graphite
Gary A. Baker, PhD, University of Missouri (Columbia)
Overview
The funding cycle from 2012-2013 represents the first year of funding for this project from the ACS PRF. This funding has made significant impact on research progress within my group, including support of one full-time graduate research assistant, one month of the PI's summer salary, and the purchase of consumables required for carrying out studies aimed at understanding ionic liquid (IL)-assisted exfoliation for graphene production.
The funding provided to this project has made a substantial impact on my student's career trajectory, as well as my own. Mr. Sudhir Ravula, who just began his third year of graduate school, has been fully supported on this ACS PRF project for the past year and has benefited immensely from having uninterrupted time in the lab. He has made significant research progress, including the completion of his first manuscript and helping to co-author a review article topically spanning his research interests. Funding for this ACS PRF project has afforded me the opportunity to generate several results aimed at understanding IL-mediated exfoliation from a computational perspective, including the mentoring of a postdoc (Dr. Ganesh Kamath) and another graduate student (Mr. Durgesh Wagle) not directly funded by this grant. A visiting undergraduate student (Ms. Wendy La, Truman State) also led nanochemistry studies which complement the ACS PRF-funded work and, although she was fully supported on a Stevens' Summer Research Fellowship, she was co-mentored by Mr. Ravula and the PI under the auspices of this project as were two local Rock Bridge High School students (Ms. Esther Liu and Ms. Prarthana Patel). Thus, the funding afforded Mr. Ravula not only the opportunity to focus on his research but also contributed to his development as a mentor, giving an undergraduate student and two high school students their first research experiences.
Scientific Progress
An atomic sheet of “chicken-wire” carbon atoms arranged in honeycomb fashion, graphene represents the thinnest material in the universe and has captured the imagination of countless scientists around to globe due to its superlative properties like giant charge mobility and record-setting thermal conductivity, stiffness, and strength. Indeed, it is widely held that graphene and its inorganic cousins (like the inorganic graphene analog (IGA) boron nitride (BN), so-called “white graphene”) are the future of electronics and the foundation for other technological revolutions including use as nanoscale building blocks for sensors and energy storage/generation devices (e.g., batteries, supercapacitors). Fully exploiting their amazing attributes requires reliable large-scale production pathways which allow retention of their inherent qualities. We recently demonstrated that ILs could assist the efficient exfoliation (molecular-scale “peeling”) of graphite into one or a handful of layers. The overarching goal of this work is to develop a molecular-level understanding of this process toward better controlling the interactions responsible for exfoliation. Two divergent roads were taken in year 1 (2012-2013) of this project in order to position us well to accomplish the scientific aims set out in our proposed project.
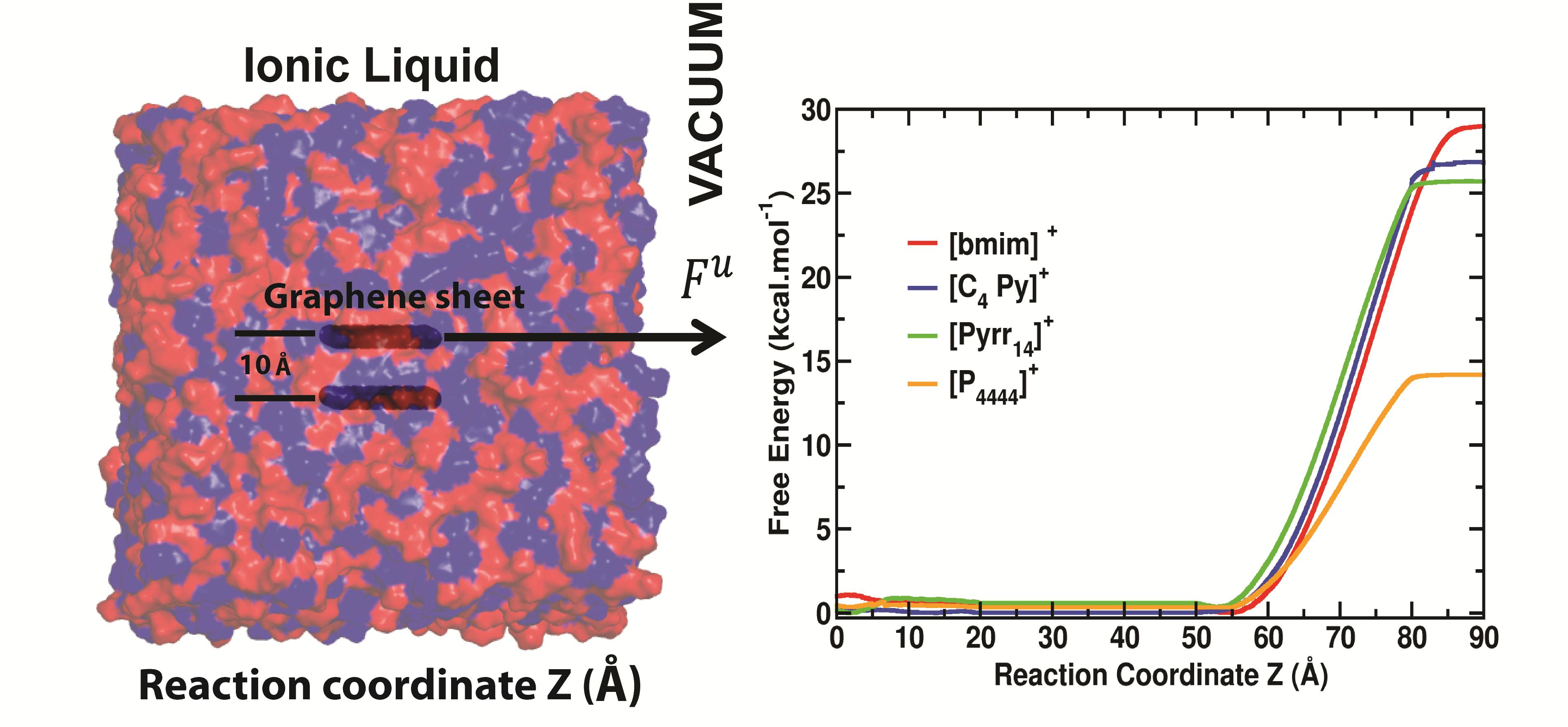
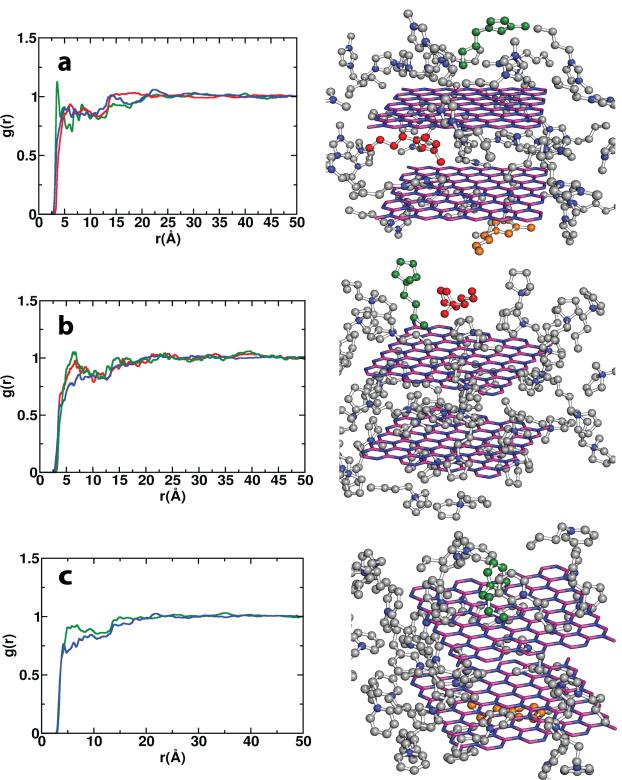
In the first area, quantum mechanical and molecular dynamics calculations formed the core of the research with an eye on elucidating the interactions occuring between IL components and graphene/IGA surfaces underpinning the exfoliation process. During year 1 of this project, this aspect included the mentoring of a postdoctoral fellow (Dr. Kamath) and a graduate student (Mr. Wagle). Four peer-reviewed journal articles have resulted from these efforts. In the first study, we employed adaptive bias force-molecular dynamics (ABF-MD) simulations to predict the free energies of exfoliation for bilayered graphene using several representative ILs (free energy solvation profiles generated by ABF-MD are shown in Figure 1), finding excellent qualitative agreement with experiment. Alkyl–π interactions with the graphene surface were found to dominate for imidazolium and pyrrolidinium ILs while π–π interactions proved more important for pyridinium ILs, characteristics previously unknown. We later extended our ABF-MD approach to the IGA boron nitride (BN). In this study, our simulations revealed a sharp contrast to the graphene results in that alkyl–π interactions with the hexagonal BN (h-BN) surface predominated whether the IL cationic backbone was of the imidazolium, pyrrolidinium, or pyridinium type (Figure 2). Moreover, the predicted free energies of exfoliation for h-BN in the ILs studied are comparable to and sometimes more favorable than that of N-methyl-2-pyrrolidinone (NMP), providing strong justification for pursuing IL-assisted exfoliation of h-BN and other layered compounds and for exploiting ABF-MD methods with other van der Waals solids.
The second research area during year 1 focused on the experimental wet chemical aspects of graphene and IGA exfoliation. In this vein, Mr. Ravula sought methods for the green exfoliation and dispersion of the IGA MoS2, of interest as a semiconductor material and for its strength, flexibility, and applications in catalysis, particularly hydrogen evolution. In this work, Mr. Ravula developed green methods for the exfoliation of single- or few-layer sheets of MoS2 in water using edible culinary agents made popular in molecular gastronomy. He has studied these materials in detail using a range of imaging and spectroscopic approaches like atomic force microscopy, transmission electron microscopy, Raman spectroscopy, and X-ray diffraction. He has also generated plasmonic hybrids built from appropriately functionalized MoS2 nanosheets aimed at catalysis and sensing. This research is currently under consideration in a high impact journal. Importantly, this work represents an entirely new line of inquiry in our group opened up by the wonderful opportunity afforded us by this ACS PRF funding.
During year 2, we will generate the first side-by-side comparisons between computational predictions for graphene/IGA exfoliation and laboratory experiments. This effort is being led by Mr. Ravula working alongside a second student in my research group, Mr. Wagle, to better understand and vet computational predictions for the same series of ILs, findings that will afford valuable insight toward designing ILs to exfoliate various nanosheets.
Figure 1. Schematic diagram for the transfer of graphene sheets from an IL-rich phase to vacuum and free energy of solvation profiles generated with ABF-MD for four different ILs.
Figure 2. Radial distribution functions describing methyl (red), butyl (green), and ring (blue) interactions between various IL cations and the h-BN surface.
Copyright © 2014 American Chemical Society