58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI752275-DNI7: Synthesis and Structure-Property Relationship Studies of Polymers Containing Core-Substituted Naphthalene Diimides
Genevieve Sauve, PhD, Case Western Reserve University
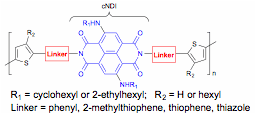
This ACS-PRF funded research explores synthesis strategies and basic structure-property relationships for polymers incorporating core-substituted naphthalene diimides (cNDI) units. Interest cNDI was revived due to advances in synthesis, easy tunability of optical properties through core substitution, and good electron transport properties. However, being small molecules, they are not easily solution-processed to make films. To improve their film-forming properties, we proposed to incorporate them into alternating copolymers through chemistry at the imide nitrogens, Figure 1. This is unlike previous work where copolymers of NDI were obtained through chemistry at the cNDI core positions. We proposed to manipulate the optical, electrochemical and electronic properties of the polymer by varying the linker, illustrated in Figure 2. We chose alkylamino cNDI because of their strong visible light absorption and fluorescence.
Figure 1. Proposed alternating copolymers.

Figure 2. Linkers and hypothesis tested in this proposal.
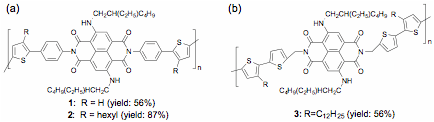
We synthesized copolymers with a phenyl linker, Figure 3a. This linker was chosen to test hypothesis 1, where the orthogonal arrangement of the units would prevent pi-stacking to give a polymer with the same optical properties as cNDI. Molecular weights tended to be small due to low conversion of the Suzuki coupling reaction and to the low solubility of these rod-like materials. The highest molecular weights were obtained using chlorobenzene and R=hexyl. The visible absorption spectra of 1 and 2 were similar to cNDI in solution, and broadened upon film formation with a bathochromic shift of ~ 20 nm. The solution emission quantum yield of 1 and 2 were 0.28 and 0.23, respectively, smaller than cNDI in solution at 0.58. Polymers 1 and 2 showed no detectable emission in films. These results are consistent with the cNDI units interacting with each other in films, although XRD of 2 does not show any diffraction peak corresponding to pi-stacking order. We surmise that the orthogonal phenyl group partly prevented pi-stacking. In the next funding period, we will use alkyl-functionalized phenyl linkers to better prevent pi-stacking.
To test our second hypothesis, we synthesized a copolymer using 2-methylene-thiophene linker, Figure 3b. The methyl group breaks the conjugation and allows more freedom for the units to pi-stack with each other. We obtained a blue-green material, 3, with a Mw of 5060, PDI=1.6 and good film-forming properties. The UV/vis absorption spectra in solution showed two absorption bands at 375 nm and 630 nm, corresponding to the tetrathiophene and the cNDI core units, respectively. Upon film formation, both absorption bands broadened, with the UV band increasing in intensity relative to the cNDI band. Emission from the cNDI unit is completely quenched in films. These results support the presence of pi-stacking interactions, 2A, though XRD does not show pi-stacking order. After analyzing calculated optimized geometry of model compounds, we realized that the dihedral angle between the thiophene and cNDI conjugated planes is 113 degree. It is therefore impossible for the polymer backbone to lay flat, as illustrated in Figure 2, hypothesis 2. On the other hand, an ethyl group allows the polymer backbone to be more planar, thus favoring better pi-stacking order. We will synthesize and study polymers with 2-ethylthiophene and other longer alkyl linkers to favor stacking interactions.
Figure 3. Synthesized polymers.
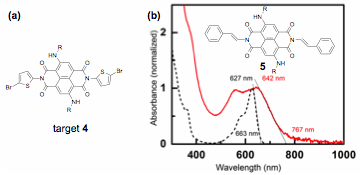
To test our third hypothesis, we attempted to synthesize monomer 4 with a thiophene at the imide position, Figure 4a. Since DFT calculations show that little energy is required to force thiophene to lay in the same plane as cNDI, we expect that a planar geometry will be favored in the solid state due to stabilization via pi-stacking. Installing a thiophene at the imide position, however, proved to be difficult. Direct imidization with 2-aminothiophene was not possible because 2-aminothiophene is unstable. Using carboxylic-acid substituted 2-aminothiophene improves stability but compromises the desired planar structure. We tried imidization with protected 2-aminothiophene or its salt, Chan-Lam coupling using 2-thiopheneboronic acid, and Stille coupling using stannylated thiophene or stannylated cNDI. During this search, we tried Chan-Lam coupling to install a vinyl group at the imide position, 5 in Figure 4b. To our surprise, the reaction worked. Moreover, a significant broadening and red-shift of the absorption spectra was observed upon film formation of 5, suggesting extension of conjugation through the imide nitrogen and/or strong pi-stacking in film. We will optimize the reaction conditions and apply our synthesis knowledge to make an alternating copolymer with a vinyl linker. We will also probe structural and charge transport properties.
Figure 4: (a) cNDI with thiophene imide substituent; (b) Synthesized cNDI with ethylene imide substituent and UV vis spectrum in solution (dash black) and film (red lie).
This ACS-PRF grant fully supported graduate student Roshan Fernando for one year, allowing him to make significant progress in his research, and giving him time and flexibility to explore difficult and risky synthesis plans, particularly with all the attempts at installing a thiophene group at the imide position. This funding also allowed undergraduate student Evan Muller to gain significant synthesis skills by working with Roshan on the project during the 2011-2012 academic year. Evan graduated in May of 2013 and is now attending Iowa State University to pursue his graduate studies. This grant partially funded research that resulted in one publication, and more publications are expected in the second year.
Copyright © 2014 American Chemical Society