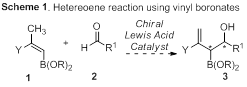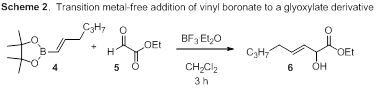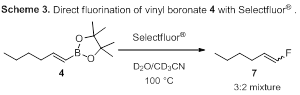58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND150647-ND1: Reactivity of Vinyl Boronates in Enantioselective Ene and Hetero-Ene Reactions
Glenn M. Sammis, PhD, University of British Columbia
Impact of research and results
The primary goal of this research project is the development of new methodology for the synthesis of functionalized small molecule, pharmaceutically relevant molecular architectures. Research during the last funding cycle began with an investigation into the development of a new vinyl boronate heteroene reaction (Scheme 1) for the synthesis of highly oxygenated small chiral building blocks, such as functionalized 1,5-diols, pyranones, and butenolides, which are ubiquitous in complex bioactive natural products and pharmaceutical agents. Initial intramolecular reactivity screens did not yield the desired product, regardless of conditions screened. To date, screens have examined the enophile, Lewis acid, solvent, and temperature (including microwave irradiation). We have continued to explore new systems to achieve the desired hetereoene reaction, including using vinyl boronates with activated allylic positions (through oxygen incorporation) and tethering strategies through the boronate ester to accelerate the heteroene reaction by enforcing proximity between the ene and enophile components.
The major competing pathway to the desired hetereoene reaction is the direct addition of the vinyl boronate into the enophile, whether it is an aldehyde or a glyoxylate (Scheme 2). Despite not being the expected product, the direct addition product is intriguing as it has typically only been observed in the presence of a transition metal. One application that we are currently pursuing is to utilize this result as the foundation for the development of new enantioselective Lewis acid-mediated transformations.
The inherent nucleophilicity of vinyl boronates also has potential applications to the direct conversion of vinyl and aryl boronates to vinyl and aryl fluorides. The importance of vinyl and aryl fluorides to both the pharmaceutical and agrochemical industries has placed a premium on the development of new synthetic methods for their preparation. There are numerous methodologies that can be used to access vinyl and aryl boronic acid. However, despite significant advances in the field, current methodologies for their direct conversion into vinyl and aryl fluorides still have limitations: such as limited substrate tolerance, large excess of reagents, or low reaction yields. A new methodology that could achieve this critical transformation would be of immense value to the pharmaceutical industry.
Initial studies of the fluorination of vinyl boronates focused on the vinyl boronate 4 (Scheme 3). A screen of solvents, electrophilic fluorine sources, and temperature revealed the highest yields of fluorinated product (7) were achieved when vinyl boronate 4 was treated with Selectfluor® in water/acetonitrile mixture at 100 °C. It is notable that the fluorinated product (7) was isolated as a 3:2 mixture of isomers.
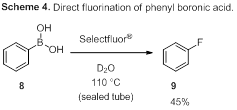
Studies then shifted to aryl boronic acids to simplify analysis by eliminating the possibility of isomer formation. A second screen of reaction conditions revealed that the highest yields of fluorinated product could be obtained when phenyl boronic acid was treated with 1.5 equivalents of Selectfluor® in water at 110 °C, to obtain the desired fluorobenzene (9, Scheme 4). However, the yields were still only moderate (up to 45%), with the protodeborylated product as the major byproduct.
We then explored the fluorination of a number of substituted arylboronic acids to get new insight into the formation of the desired aryl fluoride as well as the formation of all of the major byproducts. Our current mechanistic model for fluorination involves an SET reaction from the aryl ring to Selectfluor® followed by fluorination ipso to the boron. This two-step fluorination is consistent with our studies and also has ample precedent in other nucleophilic displacement processes with Selectfluor®. The most significant byproduct observed is the reduced product formed from direct protodeborylation. Mechanistically, this fluorination reaction differs significantly from all of the catalyst-based methods and the simplicity has the potential to allow for a general reaction. We are currently exploring strictly anhydrous conditions, electronic variations in the boronate ligands including tridentate boron ligands, external additives to chelate boron, and the addition of external additives to maximize the yield of the fluorinated product.
Impact on career
The research funding that I have received through the PRF has been of critical importance to my research career. My research group has primarily focused on the development of new radical-based synthetic methods and the application of these methods to natural product synthesis. This project has allowed my group to pursue a new research direction, new ionic reactivity. Funds from this grant have providing promising leads on heteroene reactivity using vinyl boronates, and more recently projects revolving around the inherent reactivity of sp2-boronates and sp2 boronic acids, such as the fluorination or enantioselective addition of these motifs into aldehydes. All three of these projects will lead to publications in the near future. In addition, the reactivity of vinyl and aryl boronates with electrophilic fluorine sources has attracted significant interest in the Pharmaceutical industry. This will serve as the foundation of future industrial collaborations with my laboratory. Finally, the student funding has been invaluable during the early stages of my research career.
Impact on student training
This project has been an outstanding training environment for both the graduate student and the undergraduate student who have thus far investigated the reactivity of vinyl and aryl boronates. New research directions are not just important for my career, but are of fundamental importance to the training of graduate students. It exposes the students to new and different areas of the literature and gives them additional laboratory technical skills that will prove invaluable during their future studies. In particular, this project has been very beneficial for the students involved because it has helped with their creativity in reaction development. While the initial goal has not yet been met, creative students capable of thinking outside the box have led to exciting new research results.
Copyright © 2014 American Chemical Society