58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1052588-ND10: Elucidating Competing Transport and Kinetic Mechanisms for Understanding Material Durability of Carbon Felt Electrodes
Venkat R. Subramanian, Washington University in St. Louis
1. OBJECTIVES:
There has been a significant impetus to address global environmental and energy challenges in recent years. Incorporating renewable energy sources and energy storage into the complex system of producing and distributing electricity through a smart grid are essential. Redox flow batteries promise to be a cheap and sustainable alternative for large scale energy storage. Because of the intermittent nature of many renewable energy sources (e.g. wind and solar), developing a storage system to provide a reliable and stable source of power is essential , but remains a significant challenge. Graphite felt (GF) electrodes are primarily used in redox flow batteries to provide high surface area for reaction, high electronic conductivity etc. at a reasonably low cost. However, performance and durability of these electrodes are a significant concern. Additionally, the kinetics of the redox reactions which occur on the surface of these electrodes are poorly understood and are inseparable from competing transport limitations. The primary objective of this project is to develop a detailed transport model to understand the kinetics of felt electrodes in a Thin Film Rotating Disk Electrode (TFRDE). The project also aims to develop a model to examine the surface heterogeneity and carbon corrosion that can lead to electrode damage and battery failure under high operating voltages. The insight gained from detailed interfacial studies of the GF electrode will improve understanding of the effects of various surface modification protocols reported in the literature and suggest new pathways for electrode performance and durability improvement.
2. RESEARCH FINDINGS
To understand the surface kinetics of graphite felt electrodes, a physics-based model for the TFRDE setup was developed taking into account fluid flow and material balances. The rotating disk electrode technique is a very useful and powerful technique commonly used to study electrochemical reaction kinetics. The diffusion-controlled limiting current on the RDE surface is given analytically for a steady state condition by the Levich equation:
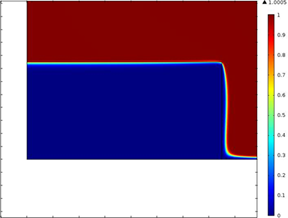
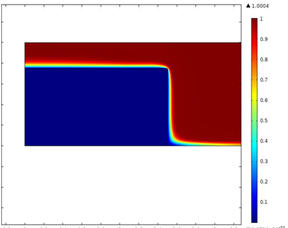
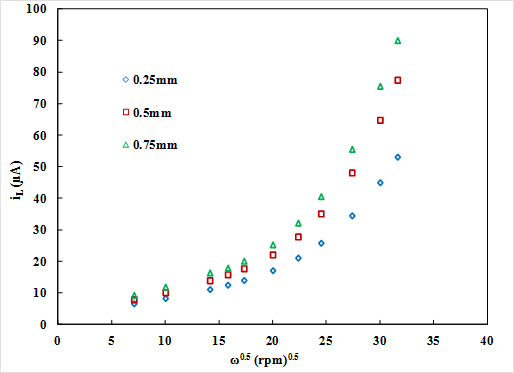
where iL is the limiting current, n is the number of electrons transferred in the half-cell reaction, F is the Faraday constant, A is the electrode area, D is the diffusion coefficient, w is the angular rotation rate of the electrode, v is the kinematic viscosity, and C is the concentration of the reactant. For the TFRDE, calculation of limiting current by the Levich equation does not match the experimental data because of the difference in flow and concentration fields as the electrolyte flows through the porous electrode film and the reaction occurs on the surface of the fibers. Currently, the competing transport and kinetic mechanisms are not properly understood. Therefore, detailed studies based on simulations are required to characterize the reactions. To address these issues, a physics based model is developed. Fig. 1 shows the modeling domain in 2 D consisting of the porous disk and electrolyte. It presents the spatial concentration of species for low and high rotation rates. At low rates, the species react on the surface of the disk while penetration in the disk is observed for higher rpms. Fig. 2 shows the variation of limiting current in the film with rotation rate as a function of film thickness. At low rotation rates, a straight line Levich-like behavior was observed. But at higher rotation speeds, the limiting current results deviate from Levich behavior and also show dependence on the thickness of electrode film. Moreover, in that region the current increases very rapidly shown by the sharp rising slope. At high rotation rates Levich like behavior is affected because of the competing effects of diffusive and convective mass transfer.
Species Concentration (mol/m3)
|
Fig. 1. Spatial variation of species for low and high rpms (A. 1000 & B. 250 rpm)
3. OUTCOMES
This research project has yielded interesting results but further studies are required to better understand the system characteristics. The solution of the detailed 2-D fluid mechanics-mass transfer coupled model and the correct mesh characteristics have been difficult to attain especially for the higher rotation speed cases. In particular, this work will indirectly result in the development of highly efficient algorithms for time stepping in fluid flow problems for porous electrodes.
4.
FUTURE RESEARCH
Fig. 2.Variation of limiting
current in the disk with rotation speed (rpm) as a function of film thickness.
Copyright © 2014 American Chemical Society