58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI152318-UNI1: The Synthesis of C4 Symmetric Oxacalix[4]arenes and Related Macrocycles
Jay Wm. Wackerly, PhD, Central College
The Wackerly research lab, and this PRF proposal, is divided into two categories: The synthesis of oxacalixarene macrocycles from electron deficient m-diphenols and the synthesis of oxacyclophane macrocycles from dihalobenzoquinones. The former is proving a more challenging project, but we have made some good progress on the latter in our first year of funding. This report will first describe our progress toward oxacyclophane macrocycles that contain benzoquinone rings and conclude with what we have learned about activating electron deficient phenols. The commonality linking these projects is that the macrocyclization reactions involve addition-elimination substitution chemistry.
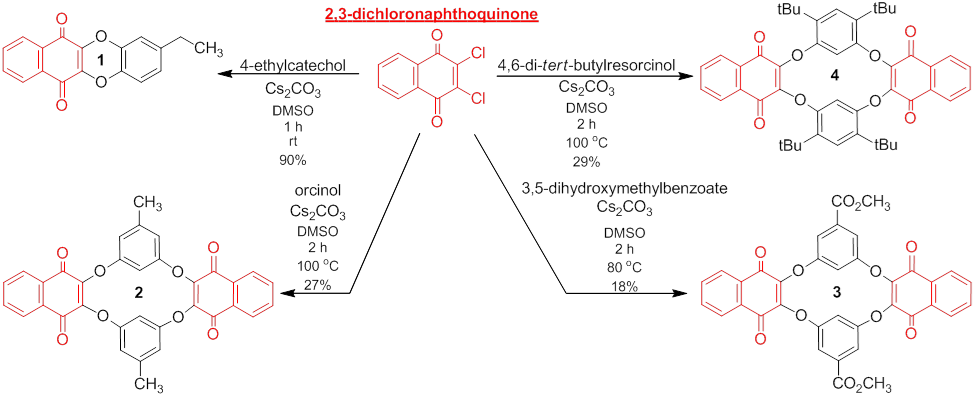
Oxaquinonacyclophanes from dichloronaphthoquinone. In my proposal I stated that our lab would investigate substitution reactions of 2,3-, 2,6-, and 2,4-dichloro-p-benzoquinones toward the synthesis of macrocyclic quinonal ethers. We selected to use 2,3-dichloronaphthoquinone as our initial electrophilic species because it is commercially available, inexpensive, and does not contain problematic hydrogens on the quinone ring. The synthesis of control compound 1 showed that we could make these cyclic ether structures and that thermodynamically and kinetically favorable ring sizes could be accessed in high yields. Though we are still optimizing the syntheses of compounds 2-4, we were able to obtain the macrocyclic tetramers in reasonable yields. Even with yields lower than ideal the macrocycles are the first compounds eluted when the mixtures are purified by column chromatography. GPC analysis of the remaining products indicates that they are larger oligomers.
Future research on this project will involve obtaining x-ray crystal structures to identify the conformations of these macrocycles and the synthesis of nucleophilic phenols of different shapes to induce larger ring sizes. Long term strategies include investigating the redox chemistry of these systems, identifying and studying macrocycles that possess a cavity for host-guest interactions, varying the geometry of the dihalo-p-benzoquinones to the 2,6- and 2,4-systems.
This research was primarily conducted by one student, Nolan Blythe '14, who presented some of this research in two poster sessions at the 2013 ACS Great Lakes Regional Meeting in LaCrosse, WI. Furthermore, I presented these results in oral and poster format at the 246th ACS National Meeting in Indianapolis, IN.
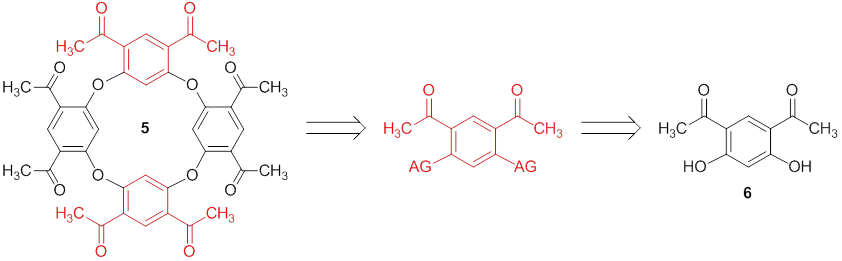
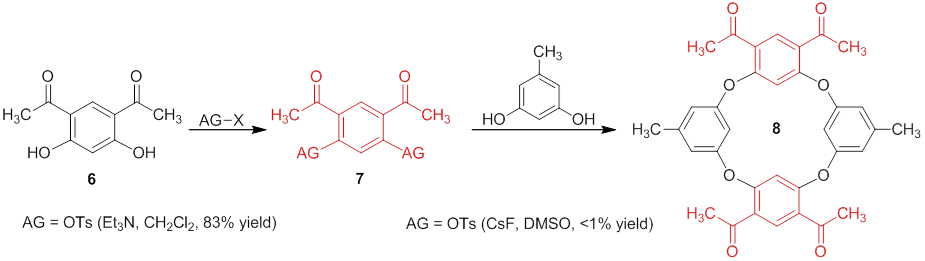
Progress towards the synthesis of a C4 symmetric oxacalixarenes. As indicated by the title, the main goal stated in this proposal was to synthesize and characterize a C4 symmetric oxacalixarene. The initial C4 target that we are identifying is oxacalixarene 5 because both the electrophilic and nucleophilic portions of the molecule can be derived from commercially available diphenol 6. To show the feasibility of this project we are first focusing our efforts on the synthesis of oxacalix[4]arene 8 because there is significant precedent illustrating that orcinol will react with electron poor m-dihaloarenes to afford oxacalixarenes under nucleophilic aromatic substitution (SNAr) conditions. We were able to synthesize and isolate macrocyle 8 in very low yield by activating diphenol 6 with tosyl groups then reacting 7 with orcinol under SNAr conditions. The yield of this reaction was, predictably, low due to preferential nucleophilic attack of the phenoxide on the tosylate sulfur which led to the predominant isolation of diphenol 6 as the major product. Our proposed goal to address this issue was to see if it was feasible to conduct a one-pot activation-substitution reaction sequence with model compound 9 to afford diaryl ether 11. After exploring reactions with five different electron poor aromatic substrates under a variety of SNAr and Ullmann coupling conditions we became convinced that the one-pot reaction is not feasible under reasonable reaction conditions (e.g., short to moderate reaction times, low to moderate temperatures, moderate to good yields). The reactions were proceeding sluggishly due to the fact that the two electron withdrawing groups make the phenol less nucleophilic. Though in some cases we could synthesize and isolate a version of 10, we concluded that it would not lead to a practical synthesis of 11 (or 8 by extension of these results). We attempted a fluorination with Xtalfluor-E, but that also did not yield 10 (AG = F). We will now turning our attention to chlorination (AG = Cl) and electron rich sulfonate esters (e.g., AG = OMs, OSO2-C6H4-p-OCH3) since there is precedent of these types of groups reacting via SNAr.
We have become convinced through our research that a one-pot approach to access oxacalixarenes from electron poor m-diphenols is not likely under SNAr or Ullman conditions with multiple strong electron withdrawing groups on a phenol (e.g., 6 and 9). We are now focusing our efforts on a two-step approach to access oxacalixarene 8 and if successful we will take a multistep approach toward the synthesis of oxacalixarene 5.
This research was primarily conducted by one student, Chase Kooyman '14, who presented some of this research in a poster sessions at the 2013 ACS Great Lakes Regional Meeting in LaCrosse, WI.
Copyright © 2014 American Chemical Society