58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR350700-UR3: The Synthesis and Characterization of Early Transition-Metal Complexes with Terminal Nitrido Ligands
Colin D. Abernethy, PhD, Sarah Lawrence College
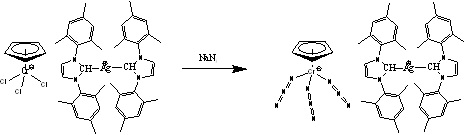
We have been seeking simple, safe and repeatable high-yield synthetic routes to early transition metal nitrido complexes containing cyclopentadienyl (Cp) co-ligands. Unfortunately, our earlier syntheses of (cyclopentadienyl)vanadium nitrido complexes have the major disadvantage of requiring the vanadium(III) sandwich compound, Cp2VCl, as the starting material (Equation 1).
Equation 1
Since Cp2VCl requires the use of highly toxic thallium salts in its synthesis (Equation 2), its preparation in multi-gram quantities is unsuitable for undergraduate research.
VCl3(thf)3 + 2CpTl → Cp2VCl + 2TlCl Equation 2
We have, therefore, sought to develop new routes to cyclopentadienyl-containing nitride complexes of both vanadium and chromium starting from either vanadocene or chromocene, which are both easily prepared by direct reaction of metal chlorides with either LiCp or NaCp.
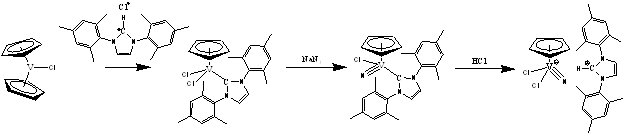
In the past twelve months, we have discovered that the reaction of either chromocene or vanadocene with excess [1,3-bis(2,4,6-trimethylphenyl)imidazole-2-ylidene]silver(I) chloride, IMesAgCl, leads to the formation of the ionic compounds [(IMes)2Ag]+[CpMCl3]- (Equation 3).
Equation 3
In this reaction, the silver(I) chloride complex: oxidizes the transition metal center from +2 to +3; provides the chloride ligands for the formation of the anionic complex [CpMCl3]-; and yields the very large cationic complex [(IMes)2Ag]+, which is ideally suited for to co-crystallize with the anionic half-sandwich complex. These reactions represent a very efficient and convenient entry point to explore the chemistry of half-sandwich complexes of both vanadium(III) and chromium(III). We therefore investigated the reactivity of the compounds [(IMes)2Ag]+[CpMCl3]- (M = V or Cr) towards azides in the hope of preparing the target (cyclopentadienyl)metal nitride complexes.
In the case of the vanadium, the reaction does, in fact, yield a compound containing an anionic (cyclopentadienyl)vanadium nitrido complex (Equation 4).
Equation 4
Now we have a safe and efficient method of preparing a compound containing the [CpV(N)Cl2]- anion we are in a great position to investigate its reactivity towards a variety of substrate molecules.
Interestingly, in the case of chromium, reaction with azide did not lead to the expected oxidation of the chromium(III) center to chromium(V) with concomitant formation of a nitride ligand, but rather an unusual anionic chromium(III) tri-azido complex formed (Equation 5).
Equation 5
We plan on using the [CpCr(N3)3]- anion as a platform on which to conduct ‘click-chemistry' in order to generate some new interesting complexes containing heterocyclic ligands.
Aspects of this research have been presented as a poster by undergraduate authors at the Canadian Society for Chemistry's national conference in Quebec City in May 2013 and an oral presentation of this work will be given at the Northeast Regional Meeting of the American Chemical Society at New Haven, CT in October.
The conduct of this work at Sarah Lawrence College has helped increase the profile of undergraduate research within the chemistry department. This has, in turn, been responsible for increasing enrollments in our upper-level chemistry courses during the past two years.
Copyright © 2014 American Chemical Society