58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1051719-ND10: New Strategies for the Preparation of Graphene Nanoribbons
Jean-Francois Morin, Universite Laval
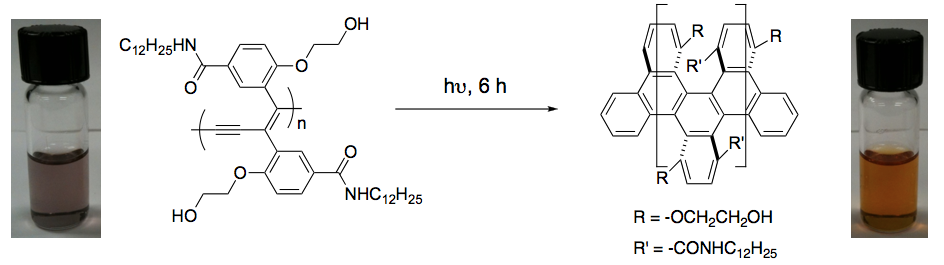
The goal of our research with this project funded by the PRF was to develop new strategies for the preparation of graphene nanoribbons using the tools of organic chemistry. The idea of using solution chemistry rather than well-known physical methods for the preparation of such materials was to have better control over the size, the shape and chemical nature of the resulting graphene nanoribbons to meet the requirement of the electronics industry in terms of materials purity, uniformity and properties. The methodology used to prepare graphene nanoribbons was not exactly the same we described in the proposal. However, we can say for sure that our methodology is by far superior in terms of synthetic feasibility and rapidity. Previous to this grant, we have developed a method to efficiently prepare aryl-appended polydiacetylenes (PDAs) for optical sensors applications. With the ACS-PRF grant money, we decided to investigate these PDAs as starting materials for the preparation of graphene nanoribbons. The initial hypothesis is that the arylenyne moieties within the PDA scaffold could be cycloaromatized to form fused aromatic systems (graphene nanoribbons) upon irradiation with UV light or gentle heating (Figure 1).
Figure 1. Synthesis of graphene nanoribbons from aryl-appended PDA
After one year and a half, we are now at determining the exact chemical nature of the materials we obtained. Although not perfect, the chemical structure of the graphene nanoribbons we obtained present unique electronic and optical properties that could be exploited in many applications. Moreover, we have undertaken the modification of the chemical functions present on the nanoribbons in order to obtain new electronic and optical properties.
In addition to nanoribbons (to be published soon), we have been able to prepare real two-dimensional graphene-like materials from long butadiyne-containing, reactive organic precursors (Figure 2). The mechanism of formation for these materials from one-dimensional initial assemblies is not yet understood and experiments are being performed to gain more insights. Nevertheless, the soluble materials we obtained possess semiconducting properties and present strong fluorescence properties. Thus, they have the potential to be used as active component in different applications, including electronics, sensors and nanomedicine. A lot of work still needs to be done to bring the synthetic methodology we developed to the multi-grams scale production and research toward this goal is still underway.
Figure 2. Synthesis of graphene-like materials from reactive precursors
The ACS-PRF New Direction grant has had a great impact in my early career. The money I got accounted for a significant part of all the grants I had from different sources in the past two years. It allows me to hire three graduate students to work on a completely new project regarding the development of new methodologies for the preparation of graphene nanoribbons and two-dimensional graphene-like materials. In only two years, this project has become the main focus of my research group with 5 graduate students now working on it full time. Without this money, I would not have started this successful project. The three students I have been able to hire with this money got a training in a highly hot topic and one of them get a job immediately after his thesis defense. The two others are completing their degrees in my research group and should be done by May 2014. Because this project is multidisciplinary, it allows the student to learn different techniques, going from chemical synthesis to materials characterization. With 2 papers published on graphene-related materials and two more submitted in the past year and a half, this project is undoubtedly a real success in term of scientific production and advancement of scientific knowledge.
Copyright © 2014 American Chemical Society